The U.S. Food and Drug Administration (FDA) has cleared GE Healthcare's artificial intelligence (AI) algorithm for reviewing critical chest x-rays.
The company's Critical Care Suite is a collection of AI algorithms embedded on its Optima XR240amx mobile x-ray system. It was built in collaboration with the University of California, San Francisco (UCSF) using GE's Edison platform.
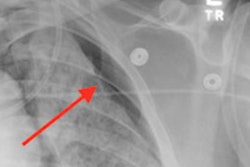
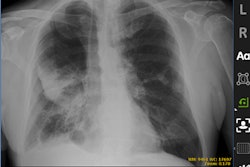
The software flags critical cases, reducing turnaround time for radiologists to review conditions such as suspected pneumothorax. It also detects acquisition errors and marks them for technologist review, so that corrections can be made before the image goes to the radiologist, GE said.