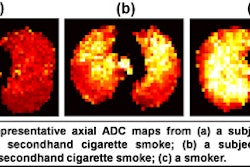
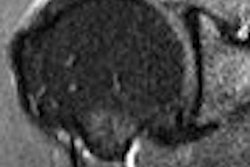
The University of Illinois at Chicago (UIC) plans to begin operation of a 9.4-tesla MRI scanner after completing human safety trials required by the U.S. Food and Drug Administration.
Data from the safety trial were published in the November issue of the Journal of Magnetic Resonance Imaging. The study included 25 healthy volunteers who were exposed to the 9.4-tesla scanner (both with static magnetic field and sodium imaging), as well as a mock scanner with no magnetic field.
There were no significant changes in heart rate, blood pressure, respiratory rate, or other vital signs when study participants were exposed to either the magnetic field or the imaging, and there were no significant differences in cognitive testing after real or mock scanning, according to the researchers.
UIC hopes the device will offer physicians a real-time view of the biological processes of the human brain.
Related Reading
Back to the present: VA updates MRI design guide, part II, December 5, 2007
Back to the present: VA updates MRI design guide, part 1, December 3, 2007
DRA vs. MRI: Why demographics will keep the modality alive, November 8, 2007
Joint Commission MR safety surveys: Moving past the fire extinguisher, August 29, 2007
Total process management for efficient MRI suites, August 8, 2007
Copyright © 2007 AuntMinnie.com