Kidney-absorbed doses of lutetium-177-based treatments for prostate cancer and neuroendocrine tumors appear to be below toxicity thresholds, according to research presented recently at the RSNA meeting in Chicago.
The finding is from a study of 93 patients receiving either Lu-177 prostate-specific membrane antigen-617 (Lu-177 PSMA-617, Pluvicto) or Lu-177 DOTATATE (Lutathera) and further highlights the need for personalized dosimetry after treatments, noted lead author Saeed Ghandili, MD, of United Theranostics, and colleagues.
“Patient-specific dosimetry for Lu-177-PSMA and Lu-177 DOTATATE therapy show overall favorable renal tolerance. But individual patient outliers emphasize the importance of routine monitoring for accurate dose management and future therapies” the researchers wrote, in a December 5 presentation.
Lu-177 PSMA-617 and Lu-177 DOTATATE are approved theranostic treatments for advanced prostate cancer and neuroendocrine tumors, respectively. Aside from targeting tumors, the radiopharmaceuticals have shown high uptake in the kidneys, which potentially puts healthy organs at risk, the authors explained.
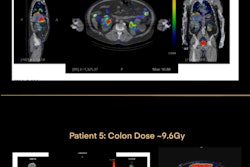
Thus, to evaluate the kidney absorbed dose in patients receiving the treatments, the researchers analyzed Lu-177 SPECT/CT scans in 93 patients -- 26 treated with Lu-77 DOTATATE and 67 treated with Lu-177 PSMA-617. The group manually segmented kidneys of the SPECT/CT scans using recently approved software at three time points for each treatment. They then calculated renal absorbed doses, with average and cumulative absorbed doses reported for each patient.
According to the analysis, all kidney-absorbed doses were below the current assumed toxicity threshold of 23 Gy.
| Cumulative absorbed kidney doses for patients (n = 93) receiving Lu-177 treatments | ||
|---|---|---|
| Dose level | Lu-177 DOTATATE | Lu-177 PSMA-617 |
| Maximum | 15.9 Gy | 18.2 Gy |
| Median | 8 Gy | 8.1 Gy |
| Minimum | 2.5 Gy | 1.1 Gy |
| Maximum dose for one patient in 1 cycle | 7.1 Gy | 9.4 Gy |
Importantly, the kidney absorbed dose increased toward later therapy cycles, the researchers noted.
“Many patients had favorable personalized renal doses, allowing for consideration of future [radiopharmaceutical therapy],” they wrote.
Also, some patients had a higher than expected personalized renal dose for a particular treatment, which highlights the importance of routine measurements for accurate cumulative dose calculation, they wrote.
Ultimately, additional efforts are needed to correlate patient-specific renal dose and other patient specific factors with kidney function over time to better define the appropriate tolerated renal dose for specific treatments, the group concluded.
The study received a magna cum laude award from the RSNA.