A new SPECT tracer based on technetium-99m (Tc-99m) is safe in patients and can detect metastatic breast cancer -- in a few cases, better than biopsies can, according to a study published August 12 in the Journal of Nuclear Medicine.
Russian investigators tested a new SPECT protein-based radiotracer, Tc-99m-(HE)3-G3, for the first time in untreated patients with breast cancer confirmed as either negative or positive for human epidermal growth factor receptor 2 (HER2) protein. They found the tracer was safe and distinguished between HER2-negative tumors and the more aggressive HER2-positive type.
"Tc-99m-(HE)3-G3 has the capability to enable visualization of metastases in multiple locations, including the bone and liver," wrote Dr. Olga Bragina, PhD, and colleagues of the Russian Academy of Sciences in Tomsk.
What's more, two patients in the trial who were diagnosed as HER2-negative by biopsies and clinical lab tests prior to participating were reassessed based on SPECT imaging and found to be HER2-positive.
"Ultimately, the tracer should be used for detection of HER2 expression in metastatic breast cancer," the group wrote.
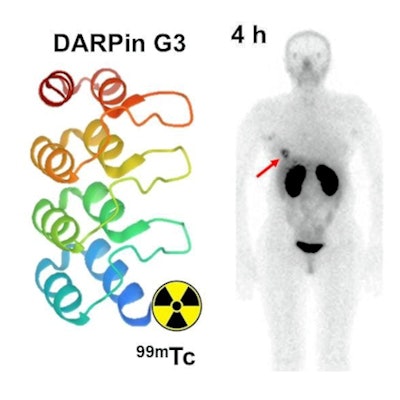
Tc-99m-(HE)3-G3 is a novel radiotracer made from a small scaffold protein with a high affinity for binding to HER2 in cancer cells. The Russian group synthesized an optimal form of the radionuclide in lab studies in 2019. In the current study, the main goal was to test the tracer for the first time in humans to evaluate its safety, biodistribution, and the most effective dose.
The researchers conducted a prospective phase-I diagnostic trial in 28 patients with untreated primary breast cancer confirmed on biopsy samples from primary tumors as either HER2-positive or HER2-negative based on American Society of Clinical Oncology guidelines.
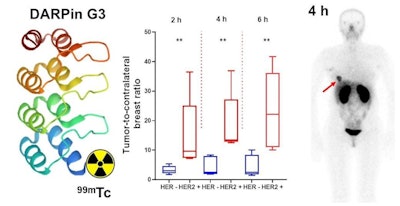 Graphical abstract. Image courtesy of the Journal of Nuclear Medicine.
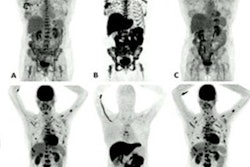
Graphical abstract. Image courtesy of the Journal of Nuclear Medicine.Three cohorts of patients with primary breast cancer (each including at least four patients with HER2-negative and five patients with HER2-positive tumors) were injected with either 1,000, 2,000, or 3,000 µg of the tracer. SPECT imaging was performed at two, four, six, and 24 hours after injection. Vital signs and possible side effects were monitored during imaging and up to seven days after injection.
No side effects were observed, the researchers reported. The tracer cleared rapidly from the blood and the highest uptake was seen in the kidneys and liver followed by the lungs, breasts, and in small intestinal content.
Liver uptake after injections with 2,000 or 3,000 µg was significantly lower than uptake after injections with 1,000 µg. Effective doses did not differ significantly between cohorts. Injections of the tracer between 2,000 and 3,000 µg would be optimal in order to suppress hepatic uptake, the group wrote.
Tumor-to-contralateral site ratios for HER-positive tumors were significantly higher than for HER2-negative tumors at two and four hours after injection. Imaging detected multiple sites of abnormal accumulation of activity. The presence of metastases at sites of abnormal accumulation in the liver and in iliac bone was confirmed by diagnostic CT images, the group wrote.
"Tc-99m-(HE)3-G3 is safe, associated with low dose burdens, and enables distinguishing HER2-positive and HER2-negative primary breast cancer lesions 2-4 hours after injection," the group stated.
In addition, high tumor-to-contralateral breast ratios on SPECT imaging triggered another look at the status of two patients enrolled as having HER2-negative tumors based on immunohistochemistry tests. In both cases, HER2-positive status was confirmed by additional testing.
"This indicates that radionuclide molecular imaging can overcome the limitations of biopsy-based methods caused by heterogeneous target expression and associated sampling errors," the researchers wrote.
Ultimately, results of the study warrant further clinical development of Tc-99m-(HE)3-G3 as a potential new tracer for SPECT imaging of HER2 expression, the group concluded.