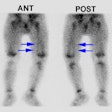
Tc-HMPAO White Blood Cell Imaging:
For Tc-HMPAO WBC imaging in specific disorders, see In-111 WBC imaging section
Chemistry and Pharmacology:
99mTc -HMPAO is lipophilic and readily crosses the cell membrane of leukocytes. Once inside the cell the compound becomes hydrophilic and remains trapped inside. It is subsequently bound to intracellular organelles, primarily the nucleus and mitochondria. The bond to granulocytes is more stable than to monocytes and the tag elutes from these cells 5 times more rapidly [1]. Others authors have claimed that the agent forms a surface complex with the white blood cell which results in early dissociation and may account for the presence of early bowel activity.In either case, unlike In-111, HMPAO is reversibly bound to leukocytes and the free agent will be excreted [4].
It is estimated that the radiation dose received by labeled cells
is at least 5 Gy which produces chromosomal damage [2]. Heavily
aberrant lymphocytes are seen in 99mTc-HMPAO labeled
mixed leukocyte preparations [2]. As a result of the DNA damage,
it is likely that the circulating aberrant lymphocytes would be
eliminated via apoptosis or phagocytosis [2]. It is unlikely that
these cells would cause a detrimental effect such as a lymphoid
malignancy [2].
The relative radiation dose from the Tc-99m WBC examination in adults is between 2.0-4.1 mSv (and 4.8-7.6 mSv when combined with sequential Tc-sulfur colloid imaging) [6].
Distribution
Compared to In-111 WBC imaging, the distribution of Tc-labeled leukocytes is more variable because some of the technetium elutes from the leukocytes and is excreted via the kidneys and hepatobiliary system resulting in urinary tract, bowel, and gallbladder activity [5]. Renal and bladder activity is usually seen due to excretion of free Tc-HMPAO (it is not related to free Technetium as the thyroid is not normally visualized) [4]. Gallbladder activity may also be identified as it is a site of HMPAO concentration via biliary excretion [4]. Physiologic bowel excretion (identified in 7% of patients on 2 hour images, and 28% of patients on 4 hour images) limits the usefulness of this agent for imaging abdominal infections. Findings which may help in differentiating physiologic bowel activity from a site of infection include: Physiologic activity is typically not seen on early images, only on delayed scans (as opposed to inflammatory bowel disease which will typically be identified on both early and 4 hour images).
Technique
The labeling procedure is similar to In-111 for adults. In children withdraw 1cc/kg of blood (20cc minimum) and use between 1mCi (minimum) and 5mCi (max.) of 99mTc -HMPAO. The labeling efficiency is between 56% to 76% [3]. Flow, Blood pool, 1 hour and 3-4 hour post injection images may be obtained (beyond 4 hours labeling starts to breakdown). Some centers perform only 2 hour post injection images.
Indications
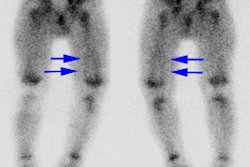
Technetium labeled leukocytes can be of value when the patient's condition requires a rapid answer and it is also good for suspected musculoskeletal infections (where renal and gut activity are not likely to mislead). In more chronic infections which may require longer periods of time before adequate tracer localization, in abdominal imaging, and in patients with FUO, Indium labeled leukocytes are probably the better choice of tracer [1]. Because of dosimetry considerations 99mTc-HMPAO labeled leukocytes are preferable for use in children.
REFERENCES:
(1) Nuclear Medicine Annual 1993; Datz FL. The current status of radionuclide infection imaging. Ed. Freeman LM. Raven Press, Ltd. New York. 47-76
(2) J Nucl Med 2002; Ak I, et al. Labeling of mixed leukocytes with 99mTc-HMPAO causes severe chromosomal aberrations in lymphocytes. 43: 203-206
(3) J Nucl Med 2004; Peacock K, et al. 99mTc-stannous colloid
white blood cell scintigraphy in childhood inflammatory bowel
disease. 45: 261-265
(4) AJR 2011; Kashefi A, et al. Molecular imaging in pulmonary
diseases. 197: 295-307
(5) J Nucl Med 2016; Palestro CJ. Radionuclide imaging of
musculoskeletal infections: a review. 57: 1406-1412
(6) AJR 2019; Sethi I, et al. Current status of molecular imaging of infection: a primer. 213: 300-308