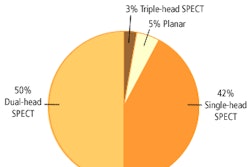
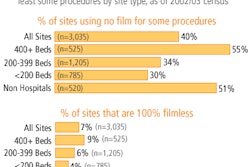
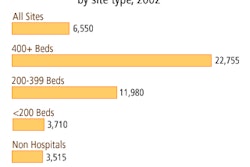
Each month the U.S. Food and Drug Administration's Center for Devices and Radiological Health (CDRH) releases a final list of devices approved for marketing in the U.S. during the previous month. The following is a list of 510(k) final decisions rendered for July 2004:
Final 510(k) decisions for July 2004
Aug 9, 2004
Latest in Industry News
Sonio taps new chief product officer
June 30, 2025
IAEA to host first Rays of Hope initiative forum
June 30, 2025