Some things are worth the wait, believes computer-aided detection (CAD) developer VuCOMP about its newly approved CAD. The company has received premarket approval (PMA) notification for its M-Vu CAD software for digital mammography.
The approval caps a long road for the company, which was one of a number of CAD developers that became ensnared in the U.S. Food and Drug Administration's (FDA) decision to change the way it regulates CAD applications. The shifting regulatory landscape led to a logjam of CAD submissions that's only begun to clear in the past couple of years.
VuCOMP now hopes to shake up a mammography CAD market that has been settled for many years between a small number of commercial products. VuCOMP will also try to differentiate the M-Vu technology from the CAD systems that are already available, according to Jeff Wehnes, the company's president and CEO.
"This is a very significant approval for us," Wehnes told AuntMinnie.com. "It allows us to truly enter the mammography CAD market in the U.S."
Beyond the black box
M-Vu's algorithms are based in part on VuCOMP's experience developing computer vision technology for military defense, according to Wehnes. The package differs from existing CAD software in that it is based on a mathematical approach to processing information and making automated decisions about the visual structures in an image, he said.
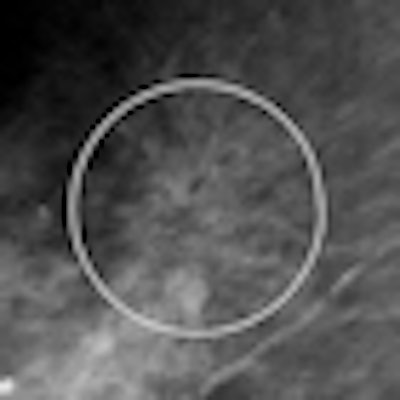
 |
| Example of VuCOMP mass detection displayed on a workstation. All images courtesy of Dick Tabbutt and VuCOMP. |
"Typical approaches to this type of problem make heavy use of 'black-box' learning technologies such as neural networks, which learn about a problem through the presentation of many examples and encode this learning in a set of numbers," he said. "With this approach, it can be very difficult for a human to understand what the black box has actually learned, which prevents an engineer from knowing how to make needed improvements during the development process. In fact, the training examples that the black box learns from inevitably have hidden biases that cause incorrect learning."
For example, consider a simplistic situation in which a developer unknowingly collects examples of noncancer cases from a clinic that overexposes its mammograms and then collects examples of cancer cases from another clinic that uses proper exposure, Wehnes said. Because a black-box algorithm learns all it knows from the examples, it might learn that overexposure means "normal" while proper exposure means "cancer"; this would negatively affect performance because the computer intelligence is reasoning improperly. And without a clear understanding of what the system has learned, the developer might never discover why the software has an unexpectedly high false-positive rate in clinical practice.
"We prefer a decision technology oriented toward the concepts of what types of visual structures appear in a mammogram," Wehnes said. "So we've constructed mathematical formulas that represent the concepts in a way that the values of the system's internal calculations map closely to our understanding of the concepts, which allows the automated intelligence to perform its reasoning in a way an engineer can interpret intuitively."
Clinical data for the digital mammography version of M-Vu included systems from Carestream Health, Fujifilm Medical Systems USA, GE Healthcare, Giotto, Hologic, Konica Minolta, Philips Healthcare, Planmed, and Siemens Healthcare; it is the first CAD study to have been approved by the FDA on such a large number of digital mammography products, according to VuCOMP.
Clinical data collected for the product's PMA submission showed both higher sensitivity and lower false-positive rates for the second-generation digital version of M-Vu compared to the original film-screen version, which was tested in a study conducted at the University of North Carolina at Chapel Hill, the company said. VuCOMP received clearance for the film-screen version of M-Vu in January 2012.
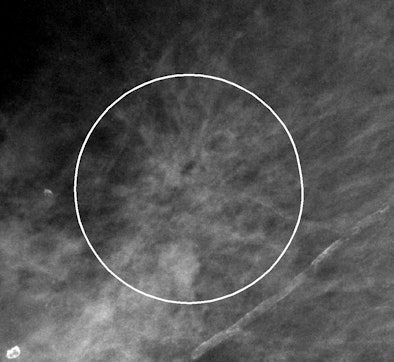 |
| Example of VuCOMP mass mark on an architectural distortion. |
A long road
Why was the road to PMA approval so long for M-Vu? In large measure, it's because the FDA in 2008 began reviewing its guidelines for how the agency regulates CAD software. The review brought approvals of new CAD applications to a halt until the backlog began clearing in 2010; the FDA only last year published final versions of its guidance to vendors.
"It became clear as we were going through the process that the kinds of studies that had been done in the past would not work, and it took a while for us to work with the FDA and determine what an acceptable study that would demonstrate effectiveness would be," Wehnes said. "We wanted to show the overall effect of the technology on the doctor's performance, not just determine whether CAD induced additional false positives. And to do this, we had to perform a reader study."
VuCOMP completed its development of a film-screen CAD algorithm in 2008, and the company conducted a clinical study to support the product in 2009. By the time the unit was approved in January, the firm had made a number of improvements and had extended the technology to digital mammography, prompting it to submit a supplemental PMA.
The marks make sense
VuCOMP plans to market M-Vu both to mammography vendors and directly to healthcare providers, according to Wehnes. The company hopes that M-Vu's ability to be used with digital mammography units from a wide array of vendors will attract practices or hospital departments that have a mix of mammography systems and want to upgrade their CAD capability.
"We have our own sales force, and we look forward to working with digital mammography vendors," he said. "If they have a customer who needs CAD, we want to help them make that happen. But we will be approaching customers directly as well."
One criticism of CAD in the past has been that the marks don't always make sense, Wehnes said.
"We have a number of U.S. installs already, and the feedback we've been getting has been overwhelmingly positive," he said. "Something we hear from doctors over and over is that they like the way M-Vu marks. Our marks don't obscure abnormalities, and they make it easy for the doctor to understand what the program is flagging for follow-up."




















