Ultrasound shows that axillary lymphadenopathy after a COVID-19 vaccine booster dose has shorter average resolution time than after an initial vaccination, according to findings published March 8 in the American Journal of Roentgenology.
A team led by Dr. Eralda Mema from Weill Cornell Medicine in New York City found that the average time to resolution for the booster dose was about 14 weeks, compared with more than 18 after initial COVID-19 vaccinations.
"Our findings provide evidence for patient counseling on expected time to resolution potentially mitigating unnecessary anxiety and mammographic screening avoidance," Mema told AuntMinnie.com.
Axillary lymphadenopathy found on breast ultrasound is a known side effect of the COVID-19 vaccine, and although it can cause concern in patients -- whether through symptoms or incidental detection on imaging -- most cases resolve on their own within about three months, the authors noted.
Mema and colleagues explored the time to resolution of vaccine-related axillary lymphadenopathy after a booster dose and evaluated what factors may be associated with resolution times via a study that included 54 patients who presented with unilateral axillary lymphadenopathy after a booster vaccine between 2021 and 2022. The condition was detected on ultrasound exams performed on either an initial breast imaging exam or follow-up prior to screening or diagnostic breast imaging; patients underwent follow-up ultrasound examinations until the lymphadenopathy resolved.
Mema's group compared time to lymphadenopathy resolution in the study cohort with time to resolution in 64 women from a previously published sample who were evaluated after an initial vaccine series.
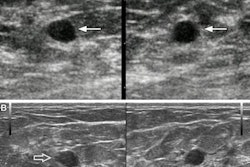
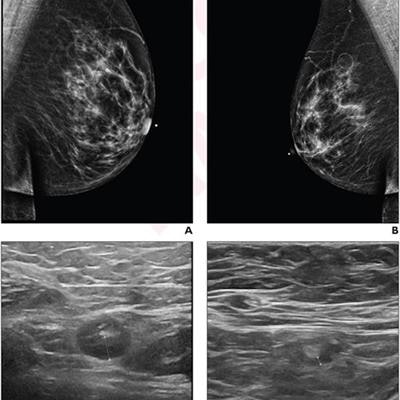
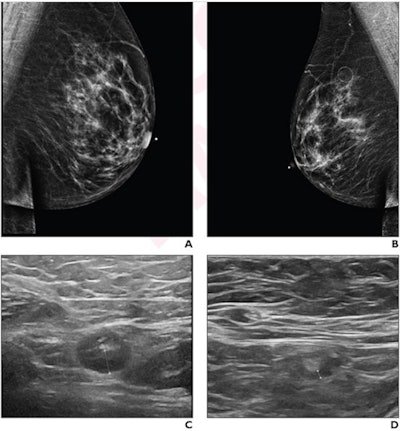 A 64-year-old woman who underwent screening mammography and breast ultrasound presented with left axillary lymphadenopathy. (A) Right and (B) left images show mediolateral oblique views that were obtained two days after a booster dose of the Pfizer COVID-19 vaccine administered in the left upper extremity shows. No right axillary lymphadenopathy was visualized. (C) Left axillary ultrasound performed on the same day as the mammogram shows an enlarged left axillary lymph node (calipers) with thickened cortex, measuring 6 mm. (D) Left axillary ultrasound performed 102 days after the booster dose shows normal lymph node (calipers) with a cortical thickness of two mm, consistent with resolution of lymphadenopathy. Images and caption courtesy of the American Roentgen Ray Society.
A 64-year-old woman who underwent screening mammography and breast ultrasound presented with left axillary lymphadenopathy. (A) Right and (B) left images show mediolateral oblique views that were obtained two days after a booster dose of the Pfizer COVID-19 vaccine administered in the left upper extremity shows. No right axillary lymphadenopathy was visualized. (C) Left axillary ultrasound performed on the same day as the mammogram shows an enlarged left axillary lymph node (calipers) with thickened cortex, measuring 6 mm. (D) Left axillary ultrasound performed 102 days after the booster dose shows normal lymph node (calipers) with a cortical thickness of two mm, consistent with resolution of lymphadenopathy. Images and caption courtesy of the American Roentgen Ray Society.The researchers found that lymphadenopathy resolved at an average of 102 days after the booster dose and 84 days after the initial ultrasound that detected it. They also found that the average time to resolution in the booster dose patients was significantly shorter than the average of 129 days seen in patients after the first dose of the initial vaccine series (p = 0.01), and that age, vaccine booster type, and history of breast cancer were not significantly associated with time to resolution.
The shorter resolution times may reflect the higher vaccine doses used for the initial Moderna vaccines (0.5 mL) compared to the booster series (0.3 mL), the group noted -- although the initial series and booster doses were the same for the Pfizer vaccine at 0.3 mL.
The time to resolution after a booster dose seen in this study supports the current recommendation for a follow-up interval of at least 12 weeks for suspected vaccine-related lymphadenopathy, Mema and colleagues explained.
Mema told AuntMinnie.com that with the rise of different COVID-19 subvariants and a reduced effectiveness of the monovalent COVID-19 vaccines, it's important to track whether there is a difference in resolution times in patients who received the monovalent versus the more recently approved bivalent COVID-19 booster.