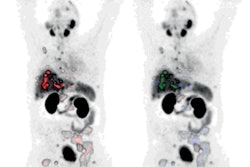
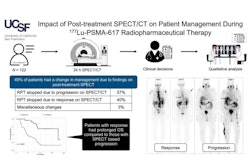
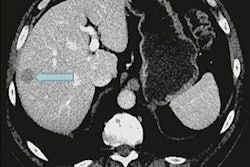
Novartis has received approval from the U.S. Food and Drug Administration (FDA) for earlier use of Pluvicto to treat patients with advanced prostate cancer.
The new indication approximately triples the eligible patient population, allowing Pluvicto to be used after one androgen receptor pathway inhibitor (ARPI) treatment and now prior to chemotherapy, Novartis said in a statement. To date, the drug has only been indicated for patients after these treatments.
“Approximately half of patients do not live long enough to receive a second treatment for [metastatic castration-resistant prostate cancer], highlighting need for earlier use of effective therapies with demonstrated tolerability,” the company said.
The expanded indication is based on the results of a phase III study in which Pluvicto reduced the risk of radiographic progression or death by 59% compared with changes in patients after treatment with ARPI therapy. In addition, Pluvicto more than doubled median radiographic progression-free survival (11.6 months vs. 5.6 months), according to the company.