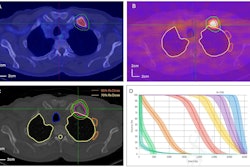
RefleXion recently received marketing clearance from the U.S. Food and Drug Administration (FDA) for the company's Scintix biology-guided radiotherapy.
The company said the therapy works for both early and late-stage cancers. It allows each cancer's unique biology to autonomously determine where and how much radiation to deliver during treatment delivery.
The company added that the therapy expands its RefleXion X1 into a dual-treatment modality platform that can treat patients with indicated solid tumors of any stage. The Scintix biologic modality tracks tumor motion from different types of tumor movement, it noted.