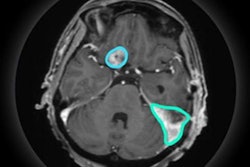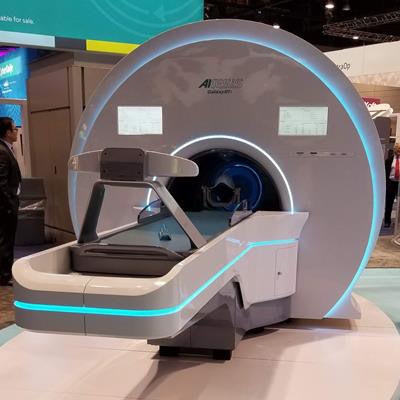
Radiation therapy developer Akesis announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Akesis Galaxy RTi gamma stereotactic radiosurgery system (SRS) for treating brain diseases and brain cancer.
Akesis Galaxy RTi is a high-precision intracranial gamma system with real-time and in-line conebeam CT and kV/kV imaging, the company said. The imaging system is mounted on a rigid ring gantry at the treatment plane, which eliminates the need to move the patient or interrupt treatment to image. It also supports fully automated intrafractional skull tracking and corrections.
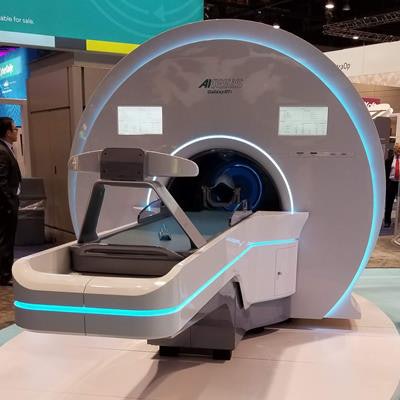 The Akesis Galaxy RTi.
The Akesis Galaxy RTi.The company's Akesis Galaxy platform has been used in more than 2,000 peer-reviewed papers for cobalt-60-based radiosurgery. This is the third system within the Akesis Galaxy SRS platform; the Akesis Galaxy RTx and Akesis Galaxy have received FDA clearance.