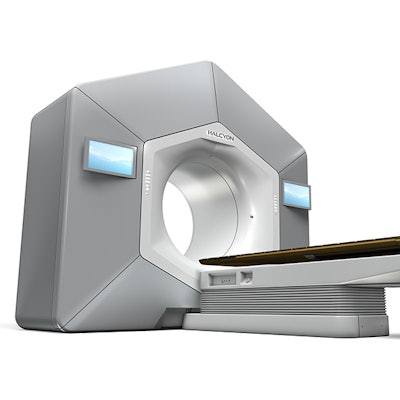
The U.S. Food and Drug Administration (FDA) has cleared radiation oncology firm Varian Medical Systems' Halcyon platform for radiation therapy.
Launched in May at the European Society for Radiotherapy and Oncology (ESTRO) meeting, Halcyon is designed to be more comfortable for patients and simpler for healthcare providers to use. The system only requires nine steps from the start of treatment to the end, compared with up to 30 steps or more with older technologies, Varian said. It features touchscreens for patient setup and has a low couch height for easier patient access.
The device requires Varian's Eclipse 15.1.1 treatment planning software, which is currently pending FDA clearance, the company said.