Breathing-induced motion can make it difficult to treat tumors in the chest and abdomen with charged particles. The main benefit of proton and heavy-ion therapy is that the beams can be "tuned" to deliver a high dose of energy at a precise location. But if the tumor shifts in and out of the beamline, or oscillates forward and backward, then the target may be missed and normal tissue irradiated instead.
Respiratory gating, in which the beam is only "on" for a certain portion of the breathing cycle, when the tumor's location is known, offers an obvious solution. What practitioners want to know, however, is whether strategies such as this are worth the extra effort. Research conducted at the National Institute of Radiological Sciences (NIRS) in Chiba, Japan, suggests that in the case of pancreatic tumors, the answer is no (International Journal of Radiation Oncology, Biology, Physics, published online September 3, 2009).
The study considered a small group of patients with pancreatic tumors who were undergoing carbon ion radiotherapy as part of an ongoing clinical trial. Pancreatic cancer is notoriously difficult to treat and the five-year survival rate is woefully poor. Particle therapy could potentially improve patients' prognosis. "Carbon ion treatment is better than photon radiotherapy because the harmful dose to normal tissue may be minimized, so the dose of chemotherapy can be increased," NIRS researcher Shinichiro Mori explained.
But should the beam "on time" be tailored to patients' breathing patterns? To answer this question, Mori and colleagues prepared two different treatment plans for seven of the patients; one that assumed continuous carbon ion delivery, and another in which the treatment beam was only on for one part of the breathing cycle.
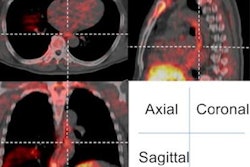
Information on the tumor's position -- and how it altered during respiration -- was acquired from 4D CT scanning. Patients were imaged while breathing freely using a rapidly rotating conebeam CT system, integrated with a 265-slice detector, in cine mode. A planning target volume was worked out for each approach that ensured the tumor would always be covered by the ion beam. Overall doses to the kidney, pancreas, duodenum, and spinal cord were calculated, as well as the dose delivered to the tumor.
The results revealed very little difference between the two approaches. In both cases, 95% of the delivered dose would have reached its intended target at all times. Nearby organs at risk generally received a lower dose when the treatment was gated, but this reduction was only small.
Given the similarity in dose distribution for the gated and ungated plans, it is probably better to stick with continuous delivery when irradiating pancreatic tumors with carbon ions, Mori and colleagues concluded. This simpler approach could have practical benefits. Gated radiotherapy could last three times longer than ungated treatment, given the breaks in ion delivery, increasing the chance of the tumor drifting out of line, or the patient's breathing getting out of sync with the predicted pattern.
So should gating be abandoned? Mori and colleagues advise caution when making such a decision. They recommend that "any application of our results to clinical use should be undertaken only after discussion with oncologists, particularly with regard to radiotherapy combined with chemotherapy."
The NIRS study of carbon ion therapy will now be extended to other abdominal organs, such as the liver, kidney, and prostate.
By Paula Gould
Medicalphysicsweb contributing editor
Oct. 27, 2009
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community Web site covering fundamental research and emerging technologies in medical imaging and radiation therapy.