The U.S. Food and Drug Administration (FDA) has approved Eli Lilly's drug donanemab (Kisunla) for the treatment of early Alzheimer's disease, including mild cognitive impairment or mild dementia.
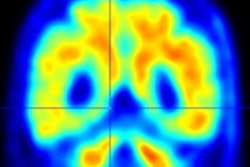
Donanemab is a monoclonal antibody that targets and clears beta-amyloid protein in the brain. It is the second drug approved by the FDA that directly targets amyloid plaque, which is believed to drive cognitive impairment. According to clinical trials, amyloid PET scans showed that donanemab reduced amyloid plaques by up to 84% after 18 months of treatment.
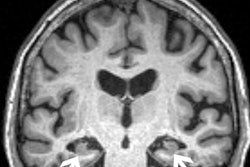
Safety warnings include that the drug can cause amyloid-related imaging abnormalities, which are detected by MRI and present as temporary swelling in an area or areas of the brain.