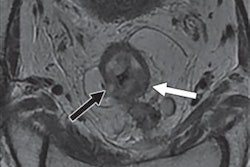
Researchers are touting the efficacy of a new, incision-free MRI-guided procedure that uses therapeutic ultrasound to treat prostate cancer and benign enlargement of the prostate gland in a multicenter study. Findings were presented Monday at RSNA 2019 in Chicago.
The outpatient procedure is known as MRI-guided transurethral ultrasound ablation (TULSA) and is designed to deliver precise doses of sound waves to diseased prostate tissue and spare healthy nerve tissue surrounding the prostate. Results from this 12-month TULSA-PRO Ablation Clinical Trial showed minimal side effects for patients and exceptional success in reducing or eliminating the cancer.
"Unlike with other ultrasound systems on the market, you can monitor the ultrasound ablation process in real-time and get immediate MRI feedback of the thermal dose and efficacy," said study co-author Dr. Steven Raman, professor of radiology and urology at the University of California, Los Angeles (UCLA) in a statement. "It's an outpatient procedure with minimal recovery time."
The company that markets the TULSA-PRO system, Profound Medical, recently received U.S. Food and Drug Administration 510(k) clearance for prostate tissue ablation. The system also is approved for clinical use in Europe.
TULSA-PRO procedures begin with the insertion into the urethra of a rod-shaped device that includes 10 ultrasound-generating elements that can cover the entire prostate gland. One or more of the components then transmits sound waves to heat and destroy the targeted prostate tissue. Software automatically controls the components to adjust the shape, direction, and strength of the therapeutic ultrasound beam. The entire procedure takes place in an MRI scanner so that clinicians can closely monitor treatment and the degree and location of heating.
For the study being presented at RSNA 2019, researchers enrolled 115 men (median age, 65; range, 59 to 69) who had median prostate-specific antigen (PSA) levels of 6.3 ng/mL (range, 4.6 to 7.9 ng/mL). The patients presented with localized low- or intermediate-risk prostate cancer, which was confined to the prostate gland. Clinicians delivered the TULSA treatment to the entire gland over an average time of 51 minutes.
Through TULSA, prostate volume among the subjects decreased on average from 39 cubic cm before the treatment to 3.8 cubic cm 12 months later. In addition, clinically significant cancer was eradicated in 80% of the patients, with 72 (65%) men showing no signs of any cancer in a biopsy after one year. Overall PSA levels also decreased by a median of 95% at one-year follow-up, and there were low rates of severe toxicity and no bowel complications.
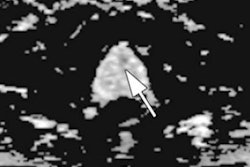
Real-time MRI thermometry and feedback control. Video courtesy of the RSNA.
"There are two very unique things about this system," added Raman, who also serves as director of prostate MR imaging and interventions and prostate MRI research at UCLA. "First, you can control with much more finesse where you're going to treat, preserving continence and sexual function. Second, you can do this for both diffuse and localized prostate cancer and benign diseases, including benign hyperplasia."
The study also supports the use of MRI for post-treatment monitoring of patients who undergo TULSA. MRI had a negative predictive value of 93% to 96% for detecting residual cancer at one year after treatment, which makes the modality very accurate for ruling out disease recurrence in patients.