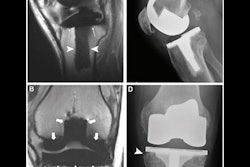
Clinicians can safely perform clinical 3-tesla MRI scans with metal artifact reduction sequence (MARS) protocols on patients with hip arthroplasty implants without excessive thermal heating of the devices, according to a study presented Thursday at the American Roentgen Ray Society (ARRS) meeting in Honolulu.
The phantom-based study concluded that the maximum temperature increase at any point was less than 2° C across all the MARS MRI pulse sequences and total hip arthroplasty devices.
"The results suggest only a minimal risk of thermal injury with the clinical MARS MRI protocols used, especially when adding the cooling effect of perfusion in physiologic conditions," wrote lead author Dr. Iman Khodarahmi and colleagues at Johns Hopkins University (JHU).
Approximately 860,000 hip and knee replacement procedures were performed by 4,755 surgeons at 654 institutions in the U.S. in 2017, according to an annual report from the American Academy of Orthopaedic Surgeons. In addition, some 2.5 million Americans have undergone total hip arthroplasty and carry on with their devices today, according to a 2014 report from the Mayo Clinic.
Given that many of these patients might have to someday undergo MRI scans, the JHU researchers set out to investigate the safety of clinical and nonclinical MARS MRI protocols on the devices at a magnet strength of 3 tesla.
For this study, Khodarahmi and colleagues took two rectangular MRI phantoms that contained a gelled saline medium to simulate the electrical and thermal properties of human muscle, along with four total hip arthroplasty implants of different designs and configurations. The devices included the following:
- One metal-on-polyethylene construct with cobalt chromium (CoCr) femoral stem
- A metal-on-metal construct with CoCr femoral stem
- Two metal-on-ceramic constructs with titanium femoral stems with two different lengths
Each implant was placed inside the phantom and at the point of maximum heat inside the scanner's bore.
Three clinical MARS MRI protocols were used in the temperature tests, including high-bandwidth turbo spin echo (HBW-TSE), slice encoding for metal artifact correction (SEMAC), and compressed sensing SEMAC (CS-SEMAC). Each protocol included six pulse sequences, with MR images obtained at coronal, sagittal, and axial angles and the range of coverage based on the length of an average patient. In addition, a 30-minute HBW sequence was modified to produce high "supraclinical" specific absorption rate (SAR) values to simulate potentially extreme MR imaging scenarios.
"Such extreme protocols are not clinically permissible," the researchers noted in their abstract, "because they exceeded the hard-coded [U.S. Food and Drug Administration] FDA limits on whole or localized body SAR and/or scanner hardware protective thresholds."
In perusing the results, the researchers found the maximum temperature increase at any one angle was 1.9° C. In most cases, the greatest increases in heating were at the tip of the femoral stem and medial aspect of the acetabular cup. In addition, they found no statistically significant difference in temperature at various points of the implant's periphery, according to a repeated measures analysis of variance (ANOVA) test (p > 0.05). They also found no statistically significant difference in temperature among the different types of implants (p > 0.05).
Even under the extreme condition of the supraclinical high SAR pulse sequences, the team recorded a temperature increase of only 3.6°C with up to 30 minutes of continuous MRI scanning.