Novartis highlighted results presented at the virtual American Society of Clinical Oncology meeting that suggest lutetium-177 (Lu-177) prostate-specific membrane antigen (PSMA)-617 is safe and effective for patients with PSMA-positive metastatic prostate cancer.
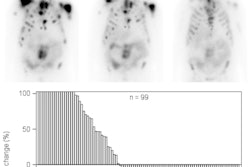
The phase-III clinical trial in men with PSMA-positive prostate cancer that has progressed despite standard treatments included more than 800 participants. Approximately 38% of men had a reduced risk of death and a 60% reduced risk of progression when treated with Lu-177 PSMA-617, according to the company.
Prostate cancer is the second most common cancer in men. More than 248,000 cases of prostate cancer and approximately 34,000 deaths are projected in the United States in 2021.
Novartis said it plans to seek approval for the targeted radionuclide therapy later this year.