PET imaging with copper-64 (Cu-64) DOTATATE could become a lower-radiation dose option for patients with suspected neuroendocrine tumors (NETs), according to the results of a phase III clinical trial published online January 10 in the Journal of Nuclear Medicine.
Developers of the radiotracer believe they have created diagnostic-quality images at a lower dose than PET using gallium-68 (Ga-68) DOTATOC and achieved similar, if not superior, rates of detection for NET lesions. Only a few minor adverse side effects were reported from the prospective study.
"In addition, diagnostic performance for Cu-64 DOTATATE PET/CT is highly reproducible and accurately identifies metastatic versus localized lesions," wrote the authors, led by Dr. Ebrahim Delpassand, founder of Excel Diagnostics and Nuclear Oncology Center in Houston. "The longer half-life, lower positron energy, and lower positron range of Cu-64 DOTATATE compared with Ga-68-labeled compounds makes Cu-64 DOTATATE a user-friendly radiopharmaceutical with the potential for practical and logistic benefits over currently approved radionuclide tracers used to identify patients with NETs."
Investigation tracer
Cu-64 DOTATATE is a diagnostic PET radiotracer designed to identify somatostatin receptors in neuroendocrine tumors. NET incidence has grown sixfold in the U.S. since 1973, due in part to better detection resulting from advances in medical imaging. Biotechnology firm RadioMedix, of which Delpassand serves as CEO, initiated the development of Cu-64 DOTATATE and signed an exclusive agreement with global nuclear medicine firm Curium in 2018 to jointly develop and commercialize the radiotracer. The two companies this month filed a new drug application (NDA) with the U.S. Food and Drug Administration (FDA).
In the current study, the companies wanted to confirm that Cu-64 DOTATATE can outperform both indium-111 (In-111) diethylenetriamine pentaacetate (DTPA) and Ga-68 DOTATOC for detecting NET lesions -- and do so at a lower radiation dose. To that end, Delpassand and colleagues conducted their single-center, prospective study to determine the sensitivity and specificity of Cu-64 DOTATATE PET/CT imaging in patients with known or suspected NETs.
Optimized dose
Their research began with a dosage study of 12 patients (mean age, 62 ± 12.7 years) to ascertain the possible lowest dose of Cu-64 DOTATATE that would produce diagnostic-quality PET/CT images. The subjects were divided into three groups of four patients: group 1 received 111 MBq (3.0 mCi), group 2 received 148 MBq (4.0 mCi), and group 3 was given 185 MBq (5.0 mCi).
PET/CT images (Biograph Horizon, Siemens Healthineers) from patients who received 148 MBq and 185 MBq doses of Cu-64 DOTATATE were deemed superior to images from the 111 MBq group. The researchers opted for a dose of 148 MBq to advance the trial, based on the as-low-as-reasonably-achievable (ALARA) principle.
The trial included 63 subjects (mean age, 54.4 ± 15.7 years) with known or suspected NETs. Of that group, 42 patients were positive for NETs and 21 were healthy volunteers. All subjects underwent a PET/CT scan an average of 63 minutes after a single intravenous dose of Cu-64 DOTATATE at 148 MBq (range, 132-163 MBq). The scan covered the skull to the midthigh in 3D mode, with an image acquisition time of five minutes per bed position over an approximately 30-minute total scan time.
Three board-certified nuclear medicine physicians evaluated the results and "categorized subjects as 'disease' or 'no disease' based only on Cu-64 DOTATATE tumor uptake," the authors explained.
Readers' results
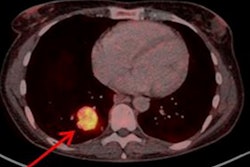
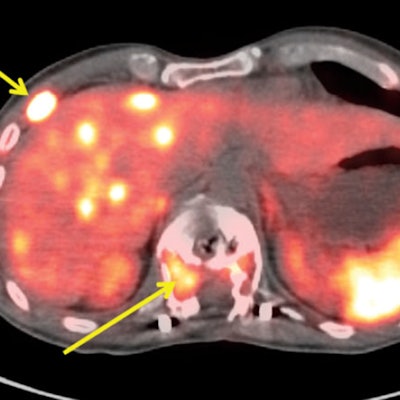
Collectively, the trio achieved sensitivity of 91%, while specificity ranged from 80% to 96% and positive predictive value ranged from 83% to 96%. Negative predictive value was 90% among the three reviewers, with accuracy ranging from 95% to 93%. The readers also differentiated between metastatic and localized disease with sensitivity and specificity of 100%, which the researchers attributed to Cu-64 DOTATATE's "excellent quality images ... to facilitate high interreader and intrareader agreement on the presence or absence of metastatic or localized disease."
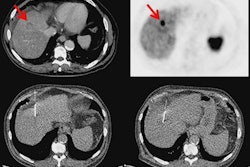
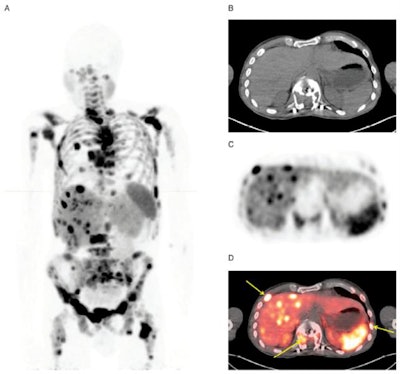 Maximum-intensity projection images with Cu-64 DOTATATE PET/CT are from a patient with metastatic bronchial carcinoid and extensive metastatic disease, including multiple small liver metastases (A). Corresponding CT image (B), PET (C), and fused PET/CT (D) show multiple bone metastases (yellow arrows). Images courtesy of JNM.
Maximum-intensity projection images with Cu-64 DOTATATE PET/CT are from a patient with metastatic bronchial carcinoid and extensive metastatic disease, including multiple small liver metastases (A). Corresponding CT image (B), PET (C), and fused PET/CT (D) show multiple bone metastases (yellow arrows). Images courtesy of JNM.Cu-64 DOTATATE prompted only a handful of minor adverse effects. Five (8%) of the 63 subjects reported a total of nine mild to moderate adverse events, with eight of those incidents deemed "probably" not related to the radiotracer. The complaints included one case each of nausea, headache, and fainting, along with two cases of vomiting.
To illustrate the clinical advantages of Cu-64 DOTATATE for NETs, Delpassand and colleagues compared their results with previous research using In-111 DTPA and Ga-68 DOTATOC. They found that Cu-64 DOTATATE's optimum 148 MBq dose was "lower than that of In-111 DTPA and similar to Ga-68-labeled radiopharmaceuticals at the commercially available [approximately] 185 MBq (5 mCi) dose."
They also touted several other characteristics of Cu-64 DOTATATE. The radiotracer is produced in a cyclotron and can be manufactured in "large-scale with a well-controlled process at a centralized location," whereas Ga-68 DOTATATE "is largely limited to major tertiary radiopharmacies with varying levels of quality control," the authors wrote. They also cited Cu-64 DOTATATE's longer half-life of 12.7 hours, compared with 1.1 hours for Ga-68 DOTATATE.
Study disclosures
Curium and RadioMedix provided funding support for the study.