Swedish and Dutch researchers are reporting early progress in combining nanoSPECT/CT with a novel pretargeted radionuclide therapy for cancer that so far is nontoxic to the kidneys, according to a preclinical study published in the July issue of the Journal of Nuclear Medicine.
For the study, the Affibody-based pretargeted peptide nucleic acid (PNA) labeled with lutetium-177 (Lu-177) was evaluated in mice bearing xenografts that express human epidermal growth factor receptor 2 (HER2). The experimental radionuclide therapy was then tested on mice in six cycles separated by seven days (JNM, July 2018, Vol. 59:7, pp. 1092-1098).
"Affibody molecules, small proteins engineered to bind to specific tumor-associated target proteins, have demonstrated excellent features for targeted molecular imaging, but their application for radionuclide therapy has so far been prevented by high renal reabsorption," said co-author Dr. Vladimir Tolmachev, a professor at Uppsala University in Uppsala, Sweden, in a statement from the Society of Nuclear Medicine and Molecular Imaging (SNMMI). The Affibody molecules have been developed by a company of the same name in Sweden.
The researchers observed rapid clearance of Lu-177 PNA from most tissues, with prominent uptake in the kidneys and the tumor. More importantly, tumor uptake was four times greater than renal uptake at one hour postinjection. In addition, 84% of the renal uptake cleared with a 15-minute half-life, compared with a tumor clearance half-life of 63 hours.
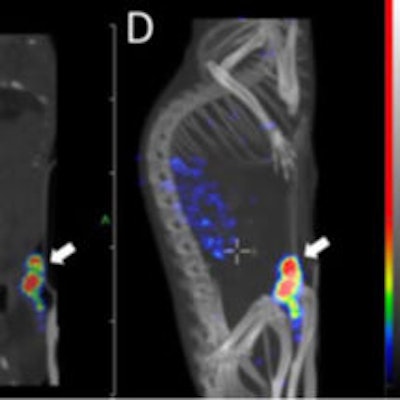
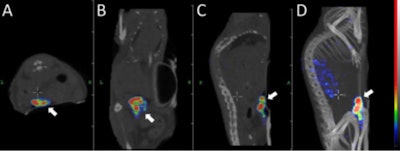 Transversal (A), coronal (B), and sagittal (C) nanoSPECT/CT and maximum intensity projection (D) images were acquired 30 hours after injection of pretargeted radionuclide therapy. The tumor (arrow) was located on the abdomen. Images courtesy of Uppsala University and JNM.
Transversal (A), coronal (B), and sagittal (C) nanoSPECT/CT and maximum intensity projection (D) images were acquired 30 hours after injection of pretargeted radionuclide therapy. The tumor (arrow) was located on the abdomen. Images courtesy of Uppsala University and JNM.The researchers concluded that the pretargeting approach could deliver an absorbed dose to tumors that appreciably exceeds the dose to critical organs, which could make Affibody-based PNA-mediated pretargeted radionuclide therapy highly efficient.
"The safe application of radiolabeled Affibody molecules for radionuclide therapy in patients will open up a whole new world of therapeutic options," said co-author Marion de Jong, PhD, a professor at Erasmus MC in Rotterdam, the Netherlands. "Affibody molecules are excellent targeting moieties and can be generated for a wide range of targets."