Researchers at Stanford University have developed a single-session PET/MR imaging protocol that reduces scan times for cancer patients and increases accuracy for evaluating chemotherapy-induced brain, heart, and bone abnormalities, according to a study published online August 4 in Radiology.
Conventional MRI protocols require 45 to 60 minutes to adequately scan the brain, heart, and bone, but the Stanford group was able to reduce imaging time to approximately 30 minute or less per organ or region. Along the way, they also uncovered incidental findings that were not part of the initial targets.
 Dr. Heike Daldrup-Link from Stanford University.
Dr. Heike Daldrup-Link from Stanford University."With children, once it gets to more than an hour of scan time, the patient starts to move and the imaging quality degrades rapidly," said study co-author Dr. Heike Daldrup-Link, PhD, professor of radiology and director of pediatric molecular imaging at Stanford University School of Medicine. "We wanted to show the feasibility that we could image the brain, heart, and joints in one scan in these patients. It took us a long time to tweak out a protocol that was satisfactory and at the same time could be done in 30 minutes or less, so we would end up with a scan of one hour to one and a half hours."
Therapy side effects
The pediatric patients in this pilot study had leukemia, lymphoma, or sarcoma and had completed treatment that included high-dose intravenous methotrexate, anthracyclines, and/or corticosteroids. Such drugs are known to have serious side effects (Radiology, August 4, 2017).
Methotrexate therapy can result in neurocognitive impairment, with an incidence rate of 48%; anthracycline therapy can induce cardiomyopathy, with an incidence rate as high as 56%; and corticosteroid therapy can lead to osteonecrosis in 10% to 22% of cases, according to the authors.
"Fortunately, for leukemia patients, there is a high survival rate, but [they] are routinely exposed to high doses of intravenous methotrexate, which can cause brain damage or bone damage," Daldrup-Link explained to AuntMinnie.com. "Patients with sarcoma generally have a very favorable outcome, but they specifically are exposed to very high doses of anthracycline, which is known to cause cardiomyopathy. That is one of the most concerning and potentially life-threatening side effects."
The challenge is also in the timing of these conditions. Osteonecrosis and bone defects, for example, can be detected at the end of therapy, but some patients develop cardiomyopathy 10 to 20 years or more after they undergo chemotherapy.
There is, however, a window of opportunity after cancer therapy in which early action could possibly ward off subsequent side effects.
"MRI can detect disease changes very early before the patient shows any symptoms. That made us think that potentially we could catch these patients early and then intervene with new therapies so we could prevent later side effects," Daldrup-Link said. "Osteonecrosis, for example, could be treated with decompression surgery. Cardiomyopathy may require a heart transplant. So obviously it will be much better if you could attack that early."
PET/MRI protocol
The researchers prospectively enrolled and scanned 10 pediatric cancer patients who had recently completed chemotherapy (mean time since therapy, 18.7 months; range, 0.5-38 months). The subjects consisted of five boys (mean age, 16 years; range, 13-19 years) and five girls (mean age, 14 years; range, 13-16 years).
The patients underwent PET/MR imaging (Signa, GE Healthcare) on a 3-tesla scanner with the aid of head, cardiac, and body coils. The scans took place one to 10 days after their clinical surveillance examination. First the head was imaged, followed by the hip and knee joints and then the heart.
One hour before imaging, the patients received an intravenous injection of FDG (2-3 MBq/kg). Head and joint MRI were performed simultaneously with PET data acquisition. Lastly, cardiac imaging was done with and without gadolinium enhancement. Postcontrast acquisition began at least 10 minutes after administration of 0.2 mL/kg of gadobenate dimeglumine (MultiHance, Bracco Imaging).
The brain MRI protocol included T2-weighted fluid-attenuated inversion-recovery (FLAIR) sequences. For cardiac imaging, cardiac short-axis steady-state free-precession cine sequences were performed to detect signs of anthracycline-induced cardiomyopathy. The bone imaging protocol included T1 fast spin-echo and short T1 inversion-recovery sequences.
Time savings
Eight of the 10 cancer patients had abnormal findings on their brain, heart, and bone images; six had clinical symptoms and two did not. The side effects included neurocognitive problems and joint pain and hip issues, as well as one patient with cardiomyopathy.
The researchers were able to complete the brain scans within 27 minutes, while bone scans were done within 15 minutes and cardiac imaging within 19 minutes. After additional training, clinicians were able to complete the PET/MRI scans within 90 minutes.
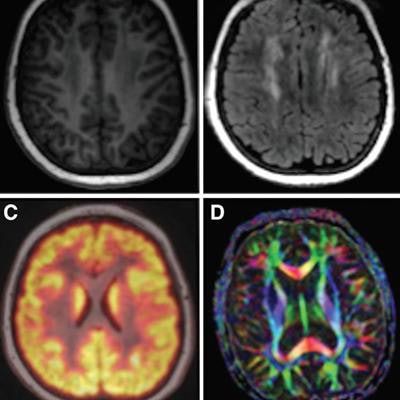
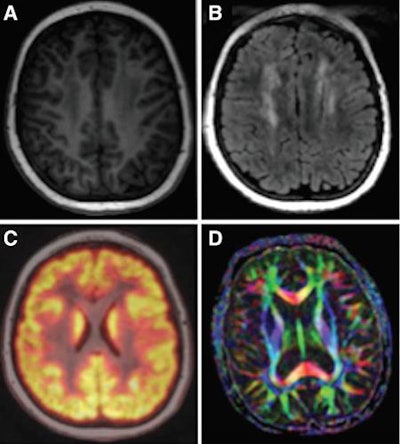 Examples of brain images include axial T1 inversion-recovery spoiled gradient-echo MR image (A); axial T2-FLAIR image (B); fused axial FDG-PET spoiled gradient-echo image (C); and axial diffusion-tensor image (D). Courtesy of Radiology.
Examples of brain images include axial T1 inversion-recovery spoiled gradient-echo MR image (A); axial T2-FLAIR image (B); fused axial FDG-PET spoiled gradient-echo image (C); and axial diffusion-tensor image (D). Courtesy of Radiology.The bone scans showed 25 osteonecrotic lesions in five patients, with all lesions detected with T1 fast spin-echo and short T1 inversion-recovery sequences. With that success, diffusion-weighted MRI and PET images could be omitted, saving scan time. With the timesaving cardiac protocol, the researchers were able to forgo T1 mapping and extracellular fractional volume calculations and shorten the time to approximately 20 minutes.
Perhaps most importantly, the researchers also discovered several incidental findings, including hip issues and brain injuries, among patients who were scanned for other reasons.
"That reinforced our thoughts that it would be good to look at the whole patient and not just that one area at the end of chemotherapy," Daldrup-Link said. "With cardiac injuries, we could confirm on the MRI scans that an increasing dose leads to increasing abnormalities in the brain and bone."
While these scans were performed on teenagers, a relatively compliant group of patients, Daldrup-Link suggested that theoretically the PET/MRI protocol could be extended to younger patients, if scans could be completed in about an hour.
Currently, the researchers are trying to set up a larger clinical trial to validate the findings with more patients. They are hoping for funding through two pending grants from the U.S. National Institutes of Health and are in discussions with Stanford affiliate Lucile Packard Children's Hospital to see if it can support such a study.