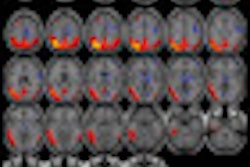
The Medical Imaging and Technology Alliance (MITA) is lauding the proposal by the U.S. Centers for Medicare and Medicaid Services (CMS) to remove the national noncoverage decision for PET with radiopharmaceuticals for U.S. Food and Drug Administration-approved oncologic applications.
If the decision is finalized, local Medicare administrative contractors may determine coverage for new oncologic agents within their respective jurisdictions for PET, according to MITA.
"We are encouraged by this first step in removing restrictions for Medicare beneficiaries to access PET procedures," said Gail Rodriguez, executive director. "A positive final decision will give cancer patients better access to cutting-edge tools physicians need to diagnose and manage cancer treatment."