Colon cancer:
General / Background:
Colorectal carcinoma is a major health problem with 130,000 to 165,000 new cases diagnosed each year in the United States [3,11]. It is the second leading cause of death from cancer in the United States and Europe [6]. At the time of diagnosis, colorectal carcinoma is localized in only 36% of patients- regional lymph node metastases are present in 39% and distant metastases are present in 19% [2]. Up to 15-20% of patients with colorectal carcinoma will have liver metastases at the time of initial surgery. Surgical resection of the primary tumor and the associated liver metastases is the only possible chance for cure [11]. Liver resection for hepatic metastases is considered only for patients with one to 4 mets confined to one lobe of the liver, and when no other adenopathy or metastases are evident (i.e.: isolated intrahepatic metastases) [3]. Synchronous adenomatous polyps are found in 30% of cases and as many as 5% of patients demonstrate synchronous colonic carcinomas [4]. About 5% of patients with colorectal carcinoma develop metachronous carcinomas at a later point in time [4]. In patients with rectal carcinoma, pulmonary metastases can occur without hepatic metastases because the hemorrhoidal vein drains into the IVC, bypassing the portal circulation.
In patients with colorectal carcinoma the expectation of cure depends on the stage of the initial tumor. Assessment of tumor extent (within and beyond the bowel wall) and the presence or absence of adenopathy are paramount for determining prognosis and the risk for tumor recurrence [3]. Primary tumors confined to the submucosa are cured in more than 90% of cases [2] and the risk for tumor recurrence is only 5% [3]. The risk for recurrence increases to 10% if the tumor invades the muscularis propria, but remains confined to the bowel wall (T2 tumor) [3]. Tumors that have extended beyond the bowel wall without lymph node involvement are cured in 60-80% of cases [5] and recurrence is seen in about 25% of cases [3]. Recurrence is seen in about 50% of cases in which the tumor has extended into neighboring structures [3]. If one to 3 lymph nodes are positive the five year survival drops to 66%, and is only 37% if four or more nodes are positive [5]. Patients with distant metastatic disease have a 5-year survival of only 13% [55]. Recurrent colorectal carcinoma occurs in 37-44% of patients within 2 years of curative resection [5]. Local recurrence at the surgical site accounts of 19%-45% of cases, whereas distant metastases account for 25%-44% [5,7]. Post-operative development of hepatic metastases is reported to occur in up to 30% of patients within 2 years of curative surgery [5].
Early detection of potentially resectable metastases or tumor recurrence can lead to improved survival [2]. Serum levels of carcinoembryonic antigen (CEA) may be used to monitor for the presence of recurrences with a sensitivity of 59%, and a specificity of 84% [2]. Unfortunately, the CEA test cannot localize the site of recurrent disease. Locoregional recurrence is more commonly encountered with rectal carcinoma, while colon carcinoma recurrence is more commonly seen in the liver or abdomen [6]. Between 15-20% of patients with locally recurrent disease are amenable to curative resection [7]. However, long term survival after attempted curative resection for metastatic or locally recurrent tumor is only about 35%- most likely due to undetected metastatic disease at other sites. Appropriate selection of patients most likely to benefit from curative surgery is crucial in order to avoid unnecessary surgery that may cause major morbidity [10].
Indications for FDG PET imaging in colorectal carcinoma include (presently reimbursed):
1- A rising CEA level in the absence of a known source: For patients with a rising CEA level, but no CT abnormality, the sensitivity of FDG PET for the detection of recurrent disease is between 93-100% and the exam can detect tumor in up to two-thirds of patients [14].
2- Equivocal lesion on conventional imaging (Evaluation for recurrent tumor for indicators other than rising CEA [i.e.: Abnormal CT scan finding])
3- Detection of hepatic and extrahepatic metastases in primary staging
4- Preoperative staging prior to resection of recurrent disease: Patients referred for the evaluation of recurrent potentially resectable colorectal carcinoma should undergo FDG PET imaging pre-operatively to exclude other sites of disease [14].
5- Distinguishing local recurrence from post-operative scar
The role of FDG PET in initial staging:
FDG PET imaging is best used to more accurately stage disease before attempted curative resection or to confirm equivocal findings on conventional imaging studies before initiating treatment [10]. One point to remember is that PET imaging is not useful for the assessment of small mucosal or submucosal tumors which are unlikely to have disseminated [1]. Also- PET is not sensitive for the detection of regional lymph node involvement (sensitivity about 29%) [35]. This is because uptake in bulky primary lesions may obscure adjacent small lymph nodes [35]. Patients with obviously disseminated disease on conventional imaging exams may not gain incremental benefit from FDG PET imaging, but the PET exam results can be used to subsequently monitor response to therapy [19].
In a prospective comparison of PET, CT, and ultrasound in the initial staging of patients with colorectal caricnoma PET was found to be the best modality for staging all sites of disease [23]. PET imaging detected 95% of the primary tumors, while CT detected only 49% [23]. For the detection of lymph node metastases, PET had a sensitivity of 25%, however, CT and ultrasound had sensitivities of 0% [23]. This is because many regional nodes in colorectal carcinoma are quite small and lie in close proximity to the primary tumor making them difficult to detect on PET imaging [25].
Metastatic disease: Another common site for metastatic disease is
the liver. Approximately 20-25% of patients present with
synchronous liver metastases, and up to 70% of all patients with
colorectal cancer will eventually develop liver mets [49,52].
Hepatic metastasectomy is used to treat liver metastases when
curative surgery is feasible [52]. However, the prognosis after
hepatecomy is poor, with a 5 year survival rate of approximately
40% [52]. The prognosis is even worse for patients with hepatic
metastatic disease at the time of presentation (synchronous mets)
[52].
For the detection of liver metastases the sensitivity, specificity, and accuracy were: PET- 78%, 96%, 91%; CT- 67%, 100%, 91%; US- 25%, 100%, 81% [23]. Other authors indicate a per lesion sensitivity for PET of 81% (compared to 74% for CT) and a per patient sensitivity of 94% (compared to 84% for CT) [49]. Lesion size is the a significant factor in the ability of PET imaging to demonstrate liver metastases [4]. Most lesions that are missed on PET imaging will be smaller than 1 cm [25] (sensitivity for lesions smaller than 1 cm is 36% [53]). Because of this, some authors recommend MR as the best modality for the evaluation of liver mets in patients that have not yet undergone therapy, but indicate that PET [49].
Studies have suggested that the SUV of the liver metastases has
greater prognostic impact than that of the primary tumor (higher
SUV within the mets is associated with a worse prognosis) and that
total lesion glycolysis and metabolic tumor volumes of the liver
mets also influence recurrence free survival and overall survival
[52]. It is interesting to note that increased metabolic activity
has been demonstrated within liver metastases following resection
of the primary tumor [41]. This has implications for the
administration of antiangiogenic therapy following surgery [41]. A
study evaluating the utility of PET/MR in colorectal cancer found
an improved 1 year recurrence free survival rate of 80% in
patients with isometabolic liver mets compared to only 14% in
patients with hypermetabolic lesions
[57]. Note that for locally advanced rectal cancer, the
SUVmax at initial staging does not predict response to
chemoradiation neoadjuvant therapy [54].
Patient management: One important point to remember is that the PET imaging findings can add incremental information which can affect patient management [25]. In a prospective study, PET findings resulted in a change in treatment modality in 8% of patients and changed the extent of surgery in 13% [23]. By identifying patients with inoperable disease, ineffective surgery can be avoided [25].
The use of PET/CT fusion imaging improves the certainty in locating and characterizing lesions in patients with colorectal cancer [24,29]. Staging and restaging accuracy improved from 78% to 89% in one study [24] and from 71% to 88% in another study [29]. However, both studies were flawed in that they compared PET/CT to PET alone (not PET reviewed with CT correlation). It is possible that side-by-side analysis of independently acquired PET and CT scans would yield a higher accuracy than PET alone [29]. The use of IV contrast for PET/CT imaging has been shown to increase the detection of hepatic metastases and for the characterization of liver lesions [43].
|
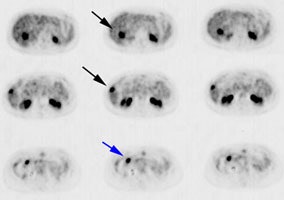
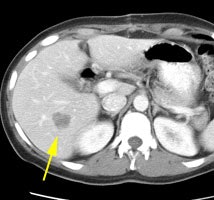
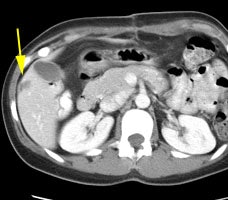
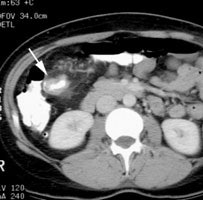
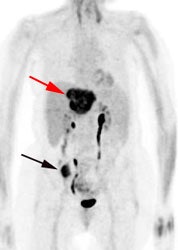
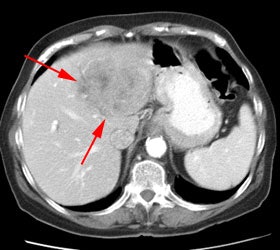
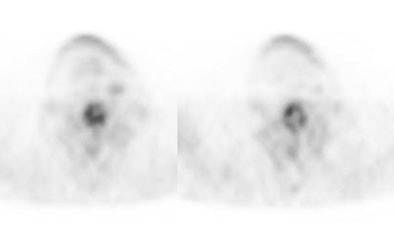
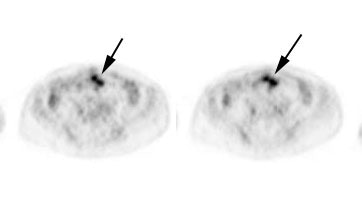
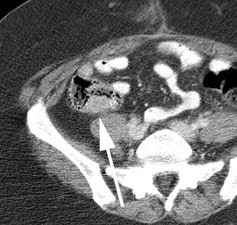
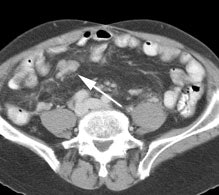
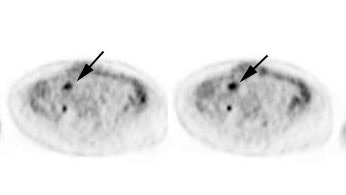
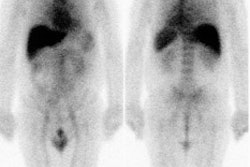
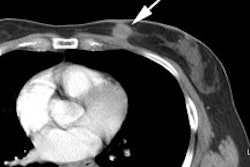
Initial staging for extrahepatic metastases: The patient below underwent FDG PET imaging to assess for extrahepatic metastatic disease prior to definitive surgical intervention. The PET exam demonstrates uptake within two hepatic metastases (black arrows on PET scan and yellow arrows on CT images) and in the patients primary colon cancer (blue arrow on PET scan and white arrow on CT image). No other sites of abnormal tracer uptake were identified which supports surgical intervention in this case. |
|
|
|
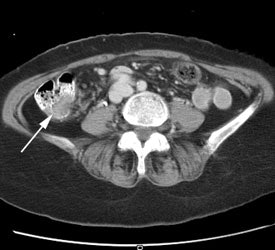
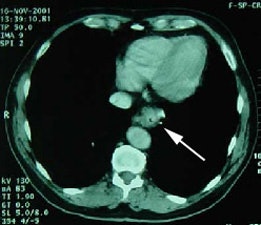
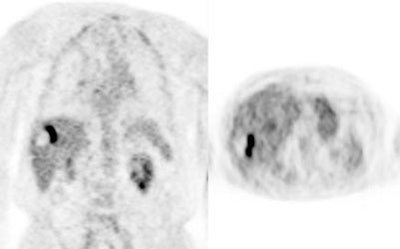
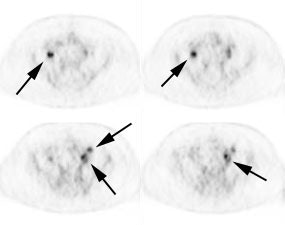
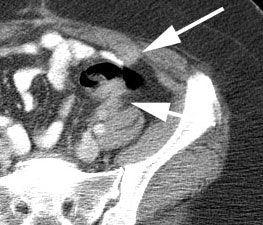
Initial staging for colorectal carcinoma: The patient below presented for evaluation of colorectal carcinoma. The CT scan demonstrated a large liver mass (red arrows) compatible with metastatic disease. The patient's cecal mass (black arrow on PET scan) was not detected on the CT study (white arrow). No other lesions were identified on the PET scan. |
|
|
Monitor response to therapy:
PET imaging can be used to monitor effectiveness of therapy. Various cutoffs in percentage decrease in SUV max have been used to differentiate responders from non-responders [44]. In general, a decrease of greater than 50-70% in SUV max is associated with response to therapy and decreased risk for recurrence [44,51]. There is a correlation between reduction in tumor FDG uptake and therapy outcome as early as 5 weeks after initiation of therapy [21]. Another study of patients receiving chemotherapy and Bevacizumab demonstrated that FDG PET/CT (using either SUV max and TLG criteria) was more accurate than conventional imaging RECIST criteria at predicting treatment response after only one cycle of therapy [51]. FDG PET imaging may also be more accurate than CT in distinguishing post-therapy changes from residual tumor in patients treated with radiofrequency ablation therapy [21,22,38]. PET imaging has the added advantage of localizing the most highly metabolic regions of the recurrent tumor which can guide biopsy if required [27].
Preoperative chemoradiotherapy is being used in patients with locally advanced colorectal carcinoma [44]. Preoperative chemoradiotherapy has been shown to reduce the incidence of local recurrence at 5 years to 6% and of distant recurrence to 36%, with an overall 5-year survival of 76% [44]. In patients receiving neoadjuvant therapy prior to planned curative resection, PET imaging can provide prognostic information and also identify unsuspected distant metastases in patients with presumed locally advanced disease [33]. A complete response on the PET scan (resolution of FDG defined disease) is associated with a good prognosis, even when post-operative specimens reveal residual tumor [33].
In rectal cancer, preoperative treatment with radiochemotherapy has been shown to produce a significant downsizing and down-staging of locally advanced tumors- thereby increasing the rate of complete surgical resection [34].Patients with a complete or even a subtotal pathologic response have an improved prognosis [34]. 18F-FDG PET imaging can be used to predict the effectiveness of pre-operative treatment as early as 12 days after beginning of treatment [34].
In radiofrequency ablation: Following RFA, leaky capillaries around the ablation site limit the ability of contrast enhanced CT or MR to reliably differentiate residual tumor [38]. Therefore, CT and MR imaging are usually delayed at least one month after RFA [38]. Ablated tumor cells lose the ability to accumulate FDG- thus, complete ablation appears as an area of photopenia [38]. Previously, it was felt that the inflammatory cellular infiltrate following RFA would generate false-positive FDG PET studies [38]. However, PET imaging performed very early following RFA (generally within 2-24 hours) would evaluate the effect of treatment before any significant inflammatory changes occurred [38]. Rim-shaped uptake following RFA is more suggestive of inflammation [38].
|
Monitoring response to radiofrequency ablation: The patient below had a remote history of radiofrequency ablation for metastatic colon cancer. The right lobe liver lesion was stable on CT scan (white arrow), but the patients CEA level was increasing. A PET scan confirmed a focus of recurrent tumor at the margin of the prior lesion. |
|
|
|
Monitoring response to radiofrequency ablation: Below is another example of a patient that had prior radiofrequency ablation for a liver metastasis from colon cancer. Note the large photopenic region which is consistent with necrotic tumor. Unfortunately, there is a large amount of residual viable tumor adjacent to this area identified on the PET scan. |
|
|
Following 90Y microsphere radioembolization there is a rapid decline in SUV in responding lesions, but not in nonresponders [48].
Evaluation for recurrence and post-operative/post-therapy change:
CEA levels are considered the marker of choice for monitoring for asymptomatic tumor recurrence [47]. A tumor recurrence is estimated in 90% of patients with an elevated CEA level [47]. When tumor recurrence is proven, the disease may be surgically resectable in 12-60% of patients [47]. Almost 50% of patients who undergo surgical resection for recurrent disease may have a long term survival of at least 80 months [47].More than half of the recurrences after curative resection of colorectal cancer are distant metastases to the liver and lungs [37]. Although an elevated CEA level is indicative of tumor recurrence, the marker provides no information regrading the site of recurrent disease. Because it provides a whole body survey, PET examinations can identify unsuspected sites of disease in patients being considered for curative resection of locally recurrent disease [42]. FDG PET/CT has been shown to have a higher sensitivity than MDCT for the identification of sitesa of rrecurrent and metasetatic disease [47]. FDG PET has very good sensitivity (89-91%) and is more accurate than CT for the detection of liver metastases [1]. FDG imaging has also been shown to be superior to conventional imaging for detecting extrahepatic metastases (sensitivity 94% versus 67% for conventional imaging) [10].
FDG PET can change patient management in 26% to 65% of cases by identifying a resectable or non-resectable metastasis that was unsuspected clinically, not seen, or equivocal on CT [1,9-12,42]. In one prospective study of 102 patients with suspected or confirmed regional recurrence of colorectal cancer, the PET imaging exam findings altered patient management in 56% of cases (excluding 6 patients in whom the primary care physician would not commit to a management plan without the PET exam results) [19]. Significantly, planned surgery was abandoned in 26 of 43 patients (60%) because of the FDG exam findings [19]. By identifying patients that would not benefit from surgery, PET imaging can result in substantial cost savings [12]. Compared to PET/CT, contrast enhanced PET/CT has been suggested to have higher accuracy and greater therapeutic impact for restaging patients with colorectal cancer [39]. In one study, by performing PET/CT as the inital imaging exam, 65% of patients would have had a clear benefit including changes in management as well as in diagnostic confidence [39]. PET imaging can also provide prognostic information as patients with recurrence that have additional lesions found on PET, compared to conventional imaging, have a worse prognosis [42]. PET imaging is clearly superior to anti-CEA SPECT imaging for the detection of distant metastases (liver, bone, and lung) and lymph node involvement when evaluating for recurrent disease [2].
|
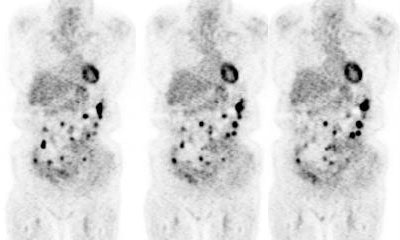
Recurrent colorectal cancer: The patient shown below had a history of colon cancer and was being evaluated for two pulmonary nodules that were suspected to be metastases. The FDG PET demonstrated uptake in the pulmonary nodules (not shown) and also revealed diffuse omental metastases. (Click image to view avi file 1.5 MB) |
|
|
Surveillance following treatment:
In one randomized study, recurrence was detected sooner in
patients in the PET group, compared to patients monitored by CT
[50]. Surgery for recurrent disease was performed more frequently
in the PET group and the frequency of curative resection of
recurrences was also higher in the PET group [50]. Fourth and
subsequent PET/CT scans obtained after completion of primary
treatment have been shown to add value to clinical assessment and
patient management decisions and to also provide prognostic
information [55]. Disease recurrence or metastatic disease can be
detected in up to 40% of patients without prior clinical suspicion
[55]. Treatment can be changed in up to 34% of patients based on
the followup PET/CT exam findings [55]. Patients with a negative
PET/CT at their fourth or subsequent exam have a better prognosis
compared to patients with positive exams [55].
Recurrence:
SUVmax, MTVtotal, and TLGtotal have been
shown to provide survival information in patients with
biopsy-proven recurrent colo-rectal cancer, especially in patients
over 59 years [56].
Local recurrence:
Primary local recurrence occurs in about 12.5% of patients [28]. Anastomotic recurrences occur in 2-4% of patients with colon cancer [46]. Anastomotic recurrence following surgery for rectal cancer is more common, but the rate is less than 10% [46]. Local and anastomic recurrence is more common in patients with poorly differentiated or advanced-stage tumors [46]. The preponderance of anastomic recurrences are reported in the literature to originate in the perianastomic soft tissues with subsequent ingrowth into the staple line [46]. Accurate differentiation of scar from tumor in the pelvis and determination of incurability in patients with suspected isolated local recurrence is crucial for proper patient management [10,26]. Unfortunately, the five year survival following salvage surgery for locally recurrent disease is only about 35% [10]. FDG imaging can be used very effectively to differentiate local recurrence from scarring following surgery/radiation therapy and has the additional benefit of providing a whole body survey which can reveal unsuspected non-local sites of recurrent disease [1,2].
Absence of tracer uptake indicates lack of disease and these patients can be followed with observation [9]. In a meta-analysis of 366 patients, the sensitivity and specificity of FDG PET for local-pelvic recurrence were 95% and 97%, respectively [21]. When compared to conventional imaging modalities, FDG PET has a sensitivity of 90-93% (versus 60% to 71% for CT/colonoscopy), a specificity of 97% (versus 72% for CT), a positive predictive value up to 88%, and a negative predictive value of 92% for the detection of local recurrence [10,21]. The accuracy of PET is between 90-100%, compared to 48-65% for CT [14,21].
False-positive FDG uptake can be seen immediately after radiation therapy due to inflammation and increased FDG uptake within irradiated tumor cells [20]. Delaying the FDG scan for at least 60 days after radiotherapy is recommended to more accurately assess response [20]. Tracer accumulation within an abnormality more than 6 months following completion of radiation therapy is strongly suggestive of tumor recurrence [14]. False positive PET exams can also occur secondary to physiologic uptake in displaced pelvic organs following surgery [26]. Use of PET/CT imaging can aid in differentiating pathologic from physiologic tracer activity [26].
|
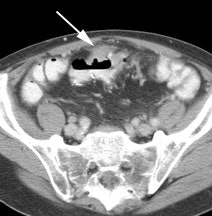
Recurrent anastomotic colorectal cancer: The patient shown below had a history of colon cancer and a rising CEA level. PET scan demonstrated abnormal tracer uptake in the abdomen (black arrows) which corresponded to an area of abnormal soft tissue thickening at the patients anastamosis on CT scan (white arrow). The patient was found to have recurrent cancer at the site of their anastamosis on endoscopy. |
|
|
Liver recurrence:
The liver is the primary site of recurrence in colorectal cancer (due to hematogenous metastases) [15,28]. After apparently curative resection of colorectal cancer, about 50% of patients will develop liver metastases within 5 years [17]. Resection of hepatic metastases is the only treatment known to improve survival in these patients [15]. Liver resection for hepatic metastases is considered for patients with one to 4 metastases confined to one lobe of the liver, that will have adequate liver reserve following resection, and no other pathologic adenopathy or metastases [16,45]. Unfortunately, only 10-20% of patients with hepatic metastases are considered surgical candidates [15]. Successful hepatic resection with curative intent is associated with a five year survival rate of about 33-40% (and a five-year disease free survival of 22%) [15,17]. Moreover, within a year of potentially curative resection up to 50% of patients show metastatic disease elsewhere [45]. The low incidence of long term disease free survival indicates that even patients considered appropriate candidates for curative resection harbor occult disease which is unrecognized at the time of surgery.
FDG PET imaging is recommended, in addition to conventional imaging, in the pre-operative evaluation of patients with potentially resectable liver metastases [40]. FDG PET imaging can be used to effectively screen candidates for curative liver resection [15]. The exam has been shown to have a very high sensitivity for the detection of liver metastases, but more importantly it also provides a whole body survey for the detection of unsuspected sites of metastatic disease [15,30]. For conventional imaging modalities, detection of colorectal hepatic metastases with CT and MR has been reported to be between 55% to 81% [15,17,30]. The sensitivity of intraoperative ultrasound has been reported to be 72% [15]. In patients with recurrent colorectal carcinoma, FDG PET has a sensitivity of 65% to 75% for the detection of individual hepatic metastatic lesions [15,17,45]. Detection of hepatic lesions on PET imaging is directly related to size, with decreased sensitivity for lesions less than 1.5 cm [17]. Although identification of individual lesions is important, it is not always necessary when determining if a patient is a surgical candidate. Appropriate characterization of individual hepatic segments as containing metastatic disease would be adequate in determining which patients are actually surgical candidates. For the detection of metastatic involvement of hepatic segments FDG PET has a sensitivity of 87% (compared to 73% for conventional imaging [CT/MR]) [15]. The use of IV contrast for PET/CT imaging has been shown to increase the detection of hepatic metastases and for the characterization of liver lesions [43]. Overall, for detection of individual lesions, contrast enhanced MR imaging is the most accurate modality [31]. However, PET imaging has the advantage of providing a whole body evaluation and can identify unsuspected extra-hepatic disease [45].
By using FDG PET imaging to evaluate patients prior to attempted curative hepatic resection a more appropriate subgroup of patients can be identified that will most benefit from this procedure and futile laparotomy can be avoided [15,18,45]. Unexpected extra-hepatic metastases leading to treatment changes can be identified in 18-43% of patients [21]. Up to 95% of patients who undergo laparotomy following staging by FDG-PET are found to have resectable disease at surgery [18]. In this same subgroup of patients, survival at 3 years improves to 77% (95% CI 60% to 94%) from about 40-45% when only conventional imaging is performed for staging prior to resection [18]. The 3 year disease free survival also increases to 40% compared to previous reported values of 15-28% [15,18]. When considering the impact of PET exam results on treatment, in two prospective studies the PET findings resulted in a change in management in 20% of patients [17]. Also- combining PET with conventional CT imaging has been shown to be the most cost effective strategy for patients presenting with metachronous liver metastases [32]. Finally, PET SUV values provide prognostic information in patients that undergo resection of liver metastases [36]. Patients with higher SUV values (above 5) had overall worse survival, despite attempted curative resection and the higher the SUV, the worse the prognosis [36].
|
Rising CEA level: The patient below had a history of treated colorectal carcinoma and a rising CEA level. The FDG PET exam demonstrated foci of abnormal tracer uptake in the liver and pelvis (black arrows). Subtle abnormalities could be identified on the CT exam and subsequent surgical exploration confirmed the presence of metastatic disease (white arrows). |
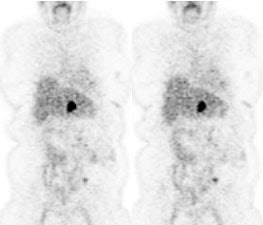 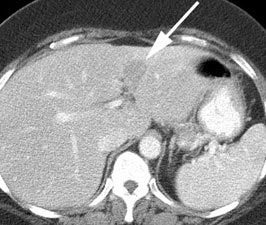
|
|
Rising CEA level: PET scan reveal positive para-aortic tracer uptake corresponding to adenopathy on CT scan consistent with recurrent colorectal carcinoma metastatic to lymph nodes. |
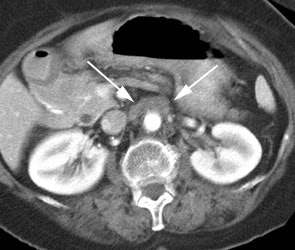 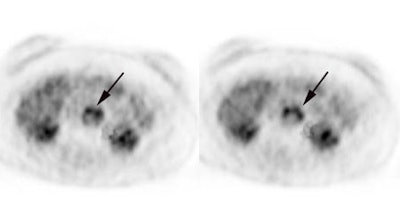
|
|
Recurrent colorectal cancer: The patient shown below had a history of colon cancer and a rising CEA level. FDG PET exam revealed a site of tracer uptake in the mesentery (black arrows) which corresponded to an enlarged lymph node seen retrospectively on CT scan (white arrow). |
|
|
Future directions of PET imaging:
18F-5-fluorouracil is biochemically identical to unlabelled 5-fluorouracil and can be used to assess for drug concentration in target areas prior to the initiation of treatment [20]. Patients with high tumor 18F-5-fluorouracil SUV's (greater than 2.5) are more likely to achieve a response and survive longer than patients with lower 18F-5-fluorouracil uptake [20].
Limitations of FDG PET imaging in colorectal carcinoma:
As with other cancers, FDG PET imaging may fail to detect small volume disease [9] due to partial-volume averaging with small lesions (under 1 cm) or in necrotic lesions with only a thin rim of viable tissue [1,10]. FDG imaging has also been shown to be less sensitive for the detection of mucinous carcinoma (sensitivity 58% compared to 92% for non-mucinous lesions), possibly due to a relative hypocellularity [10,23]. Normal colorectal tracer activity may potentially mask a lesion [23].
False-positive exams occur in only about 4% of patients [10]. This is because occasionally, normal gastrointestinal tract accumulation of the tracer may be difficult to differentiate from a malignant lesion [1]. Uptake at sites of prior anastamosis has also been described [10]. All extrahepatic foci of FDG PET accumulation should be evaluated with a CT scan through the region of abnormality to confirm the presence of a lesion and evaluate its resectability [1].
Following radiation therapy, FDG accumulation may be related to radiation induced inflammation. Delaying PET studies for 2 to 3 months following completion of radiation therapy is recommended for more accurate imaging [21]. By 6 months following XRT, any activity noted on PET FDG exams should be considered consistent with tumor recurrence [1].
Increased glucose metabolism can be seen in colonic adenomas which are considered to be pre-malignant lesions [13]. Up to 90% of adenomas larger than 1.3 cm can be identified on FDG imaging [13].
REFERENCES:
(1) J Nucl Med 1999; Delbeke D. Oncological applications of FDG PET imaging: Brain tumors, colorectal cancer, lymphoma, and melanoma. 40: 591-603
(2) J Nucl Med 2000; Willkomm P, et al. FDG PET and immunoscintigraphy with 99mTc-labeled antibody fragments for detection of recurrence of colorectal carcinoma. 41: 1657-1663
(3) Radiol Clin North Am 1997; Thoeni RF. Colorectal cancer. Radiologic staging. 35: 457-485
(4) Radiology 2000; Levine MS, et al. Diagnosis of colorectal neoplams at double-contrast barium enema examination. 216: 11-18 (No abstract available)
(5) Radiographics 2000; Horton KM, et al. Spiral CT of colon cancer: Imaging features and role in management. 20: 419-430
(6) J Am Coll Surg 2000; Lechner P, et al. Can postoperative surveillance with serial CEA immunoscintigraphy detect resectable rectal cancer recurrence and potentially improve tumor-free survival? 191: 511-518
(7) Annals of Surgery 1997; Hughes K, et al. Use of carcinoembryonic antigen radioimmunodetection and computed tomography for predicting resectability of recurrent colorectal carcinoma. 226: 621-631
(8) Clin Nucl Med 1999; Yoshoka T, et al. FDG PET evaluation of residual masses and regrowth of abdominal lymph node metastases from colon cancer compared with CT during chemotherapy. 24: 261-263 (No abstract available)
(9) Clin Pos Imag 2000; Kalff V, et al. F-18 FDG PET for suspected or confirmed regional recurrence of colon cancer: A prospective study of impact and outcome. 3: 183
(10) Dis Colon Rectum 2000; Whiteford MH, et al. Usefulness of FDG-PET scan in the assessment of suspected metastatic or recurrent adenocarcinoma of the colon and rectum. 53: 759-70
(11) Am Surg1999; Boykin KN, et al. The use of FDG-positron emission tomography for the evaluation of colorectal metastases of the liver. 65: 1183-85
(12) J Nucl Med 2001; Meta J, et al. Impact of 18F-FDG PET on managing patients with colorectal cancer: The referring physician's perspective. 42: 586-590
(13) J Nucl Med 2001; Yasuda S, et al. 18F-FDG PET detection of colonic adenomas. 42: 989-992
(14) Radiol Clin N Am 2001; Delbeke D, Martin WH. Positron emission tomography in oncology. 39: 883-917
(15) AJR 2002; Rydzewski B, et al. Usefulness of intraoperative sonography for revealing hepatic metastases from colorectal cancer in patients selected for surgery after undergoing FDG PET. 178: 353-358
(16) Radiol Clin North Am 1997; Thoeni RF. Colorectal cancer. Radiologic staging. 35: 457-485
(17) J Clin Oncol 2002; Ruers TJM, et al. Value of positron emission tomography with [F-18]Fluorodeoxyglucose in patients with colorectal liver metastases: a prospective study. 20: 388-395
(18) Ann Surg 2001; Strasberg SM, et al. Survival of patients evaluated by FDG-PET before hepatic resection for metastatic colorectal carcinoma: a prospective database study. 233: 293-299
(19) J Nucl Med 2002; Kaliff V, et al. The clinical impact of 18F-FDG PET in patients with suspected or confirmed recurrence of colorectal cancer: a prospective study. 43: 492-499
(20) J Nucl Med 2003; Kostakoglu L, Goldsmith SJ. 18F-FDG PET evaluation of the response to therapy for lymphoma and for breast, lung, and colorectal carcinoma. 44: 224-239
(21) Radiographics 2003; Kostakoglu L, et al. Clinical role of FDG PET in evaluation of cancer patients. 23: 315-340
(22) Clin Nucl Med 2003; Anderson GS, et al. FDG positron emission tomography in the surveillance of hepatic tumors treated with radiofrequency ablation. 28:192-7
(23) J Nucl Med 2003; Kantorova I, et al. Routine 18F-FDG PET preoperative staging of colorectal cancer: comparison with conventional staging and its impact on treatment decision making. 44: 1784-1788
(24) J Nucl Med 2003; Cohade C, et al. Direct comparison of 18F-FDG PET and PET/CT in patients with colorectal carcinoma. 44: 1797-1803
(25) Radiology 2004; Rohren EM, et al. Clinical applications of PET in oncology. 231: 305-332
(26) Radiology 2004; Even-Sapir E, et al. Detection of recurrence in patients with rectal cancer: PET/CT after abdominoperineal or anterior resection. 232: 815-822
(27) AJR 2005; Barker DW, et al. Evaluation of liver metastases after radiofrequency ablation: utility of 18F-FDG PET and PET/CT. 184: 1096-1102
(28) Radiol Clin N Am 2004; Hustinx R. PET imaging in assessing gastrointestinal tumors. 42: 1123-1139
(29) J Nucl Med 2005; Kim JH, et al. Comparison between 18F-FDG PET, in-line PET/CT, and software fusion restaging of recurrent colorectal cancer. 46: 587-595
(30) AJR 2005; Sahani DV, et al. Detection of liver metastases from adenocarcinoma of the colon and pancreas: comparison of mangafodipir trisodium-enhanced liver MRI and whole-body FDG PET. 185: 239-246
(31) Radiology 2005; Bipat S, et al. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis- meta-analysis. 237: 123-131
(32) J Nucl Med 2005; Use of a decision analysis model to assess the cost-effectiveness of 18F-FDG PET in the management of metachronous liver metastases of colorectal cancer. 46: 2020-2028
(33) J Nucl Med 2006; Kalff V, et al. Findings on 18F-FDG PET scans after neoadjuvant chemoradiation provides prognostic stratification in patients with locally advanced rectal caricnoma subsequently treated by radical surgery. 47: 14-22
(34) J Nucl Med 2006; Cascini GL, et al. 18F-FDG PET is an early predictor of pathologic tumor response to preoperative radiochemotherapy in locally advanced rectal cancer. 47: 1241-1248
(35) Radiology 2007; Blodgett TM, et al. PET/CT: form and function. 242: 360-38
(36) J Nucl Med 2007; Riedl CC, et al. 18F-FDG PET scanning correlates with tissue markers of poor prognosis and predicts mortality for patients after liver resection for colorectal metastases. 48: 771-775
(37) AJR 2007; Choi YJ, et al. CT colonography for followup after surgery for colorectal cancer. 189: 283-289
(38) AJR 2007; Khandani AH, et al. A pilot study of early 18F-FDG PET to evaluate the effectiveness of radiofrequency ablation of liver metastases. 189: 1199-1202
(39) J Nucl Med 2008; Soyka JD, et al. Staging pathways in recurrent colorectal carcinoma: is contrast-enhanced 18F-FDG PET/CT the diagnostic tool of choice? 49: 354-361
(40) J Nucl Med 2008; Fletcher JW, et al. Recommendations on the use of 18F-FDG PET in oncology. 49: 480-508
(41) J Nucl Med 2008; Scheer MGW, et al. Increased metabolic activity of indolent liver metastases after resection of a primary colorectal tumor. 49: 887-891
(42) J Nucl Med 2008; Scott AM, et al. PET changes management and improves prognostic stratification in patients with recurrent colorectal cancer: results of a multicenter prospective study. 49: 1451-1457
(43) AJR 2008; Badiee S, et al. Role of IV iodinated contrast material in 18F-FDG PET/CT of liver metastases. 191: 1436-1439
(44) J Nucl Med 2009; Ben-Haim S, Ell P. 18F-FDG PET and PET/CT in the evaluation of cancer treatment response. 50: 88-99
(45) J Nucl Med 2009; Ruers TJM, et al. Improved selection of
patients for hepatic surgery of colorectal liver metastases with
(46) AJR 2010; Shyn PB, et al. PET/CT pattern analysis for surgical staple line recurrence in patients with colorectal cancer. 194: 414-421
(47) AJR 2010; Metser U, et al. Assessment of tumor recurrence in patients with colorectal cancer and elevated carcinoembryonic antigen level: FDG PET/CT versus contrast-enhanced 64-MDCT of the chest and abdomen. 194: 766-771
(48) Radiology 2010; Tochetto SM, et al. Does multidetector CT attenuation change in colon cancer liver metastases treated with 90Y help predict metabolic activity at FDG PET? 255: 164-172
(49) Radiology 2010; Niekel MC, et al. Diagnostic imaging of
colorectal liver metastases with CT, MR imaging, FDG PET, and/or
PET/CT: a meta-analysis of prospective studies including patients
who have not previously undergone treatment. 257: 674-684
(50) J Nucl Med 2013; Patel K, et al. The lack of evidence for
PET or PET/CT surveillance of patients with treated lymphoam,
colorectal cancer, and head and enck cancer: a systemic review.
54: 1518-1527
(51) J Nucl Med 2013; Lastoria S, et al. Early PET/CT scan is
more effective than RECIST in predicting outcome of patients with
liver metastases from colorectal cancer treated with preoperative
chemotherapy plus bevacizumab. 54: 2062-2069
(52) J Nucl Med 2014; Lee HS, et al. Prognostic value of
metabolic parameters in patients with synchronous colorectal
cancer liver metastasis following curative-intent colorectal and
hepatic surgery. 55: 582-589
(53) Radiographics 2014; Tirumani SH, et al. Update on the role
of imaging in management of metastatic colorectal cancer. 34:
1908-1928
(54) AJR 2015; Maffione AM, et al. Value of 18F-FDG
PET for predicting response to neoadjuvant therapy in rectal
cancer: systematic review and meta-analysis. 204: 1261-1268
(55) J Nucl Med 2015; Marcus C, et al. 18F-FDG PET/CT
and colorectal cancer: value of fourth and subsequent posttherapy
followup scans for patient management. 56: 989-994
(56) AJR 2016; Marcus C, et al. Value or quantitative FDG PET/CT
volumetric biomarkers in recurrent colorectal cancer patient
survival. 207: 257-265
(57) Radiology 2016; Lee DH, et al. Colorectal cancer liver
metastases: diagnostic performance and prognostic value of PET/MR
imaging. 280: 782-792