PET/CT imaging in patients with metastatic melanoma who receive immunotherapy drugs can show which patients are more or less likely to survive, according to a study published December 12 in the European Journal of Radiology.
A group of Austrian and Iranian researchers analyzed data from F-18 FDG-PET/CT scans in patients before and after they began immunotherapy with one of three currently approved drugs. They found PET/CT scans performed a few months after patients began therapy predicted three- and five-year overall survival rates.
"Baseline and follow-up imaging biomarkers served as surrogate markers of shorter overall survival in patients with melanoma undergoing immunotherapy," wrote corresponding author Mohsen Beheshti, PhD, head of molecular imaging and theranostics at Paracelsus Medical University in Salzburg, Austria.
Malignant melanoma accounts for less than 5% of cutaneous malignancies, yet it is the most lethal subtype of skin cancer. New immunotherapy drugs, such as ipilimumab, nivolumab, and pembrolizumab have become a dominant strategy in the treatment of advanced melanoma in the last decade, and they have achieved unprecedented improvements in patient survival, according to the study authors.
However, despite such promising results, these treatments are expensive and associated with significant potential toxicities, Beheshti and colleagues said.
"The early noninvasive risk-stratification of patients undergoing immunotherapy is crucial," they wrote.
Previous studies have shown that F-18 FDG-PET/CT can evaluate whether patients with malignant melanoma will respond to immunotherapy, yet few investigations have explored the use of F-18 FDG-PET/CT as a prognostic tool for determining their overall survival.
In this retrospective study, the researchers included 51 patients with metastatic melanoma who had begun immunotherapy between October 1, 2011, and November 30, 2017. Patients had baseline whole-body F-18 FDG PET/CT scans (Philips TF Ingenuity or Siemens Healthineers Biograph mCT) and at least two follow-up scans at three and six months after they began therapy.
The researchers extracted multiple metabolic parameters, including FDG standardized uptake values and tumor-to-background ratios (TBRs), from the scans and analyzed them against overall survival rates. The overall survival rate after three years was 49% and 43.1% after five years.
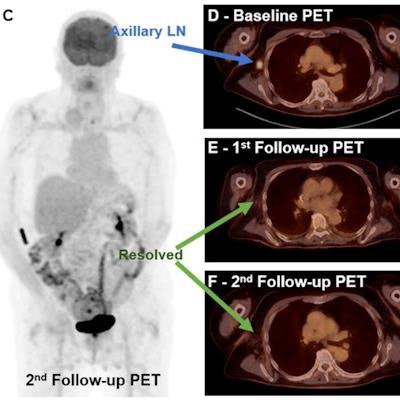
![Complete metabolic response to therapy. A 72-year-old male with metastatic melanoma. Maximum intensity projection (MIP) of baseline (A) and transaxial fused (D) F-18 FDG-PET/CT images show a hypermetabolic right axillary lymph node (blue arrow), which is resolved on both first (B [MIP] and E [fused transaxial]) and second (C [MIP] and F [fused transaxial]) follow-up studies (green arrows). Image courtesy of the European Journal of Radiology.](https://img.auntminnie.com/files/base/smg/all/image/2021/12/am.2021_12_15_01_22_4554_2021_12_15_FDG_uptake_melanoma.png?auto=format%2Ccompress&fit=max&q=70&w=400) Complete metabolic response to therapy. A 72-year-old male with metastatic melanoma. Maximum intensity projection (MIP) of baseline (A) and transaxial fused (D) F-18 FDG-PET/CT images show a hypermetabolic right axillary lymph node (blue arrow), which is resolved on both first (B [MIP] and E [fused transaxial]) and second (C [MIP] and F [fused transaxial]) follow-up studies (green arrows). Image courtesy of the European Journal of Radiology.
Complete metabolic response to therapy. A 72-year-old male with metastatic melanoma. Maximum intensity projection (MIP) of baseline (A) and transaxial fused (D) F-18 FDG-PET/CT images show a hypermetabolic right axillary lymph node (blue arrow), which is resolved on both first (B [MIP] and E [fused transaxial]) and second (C [MIP] and F [fused transaxial]) follow-up studies (green arrows). Image courtesy of the European Journal of Radiology.Results from baseline F-18 FDG-PET/CT scans showed uptake values (wSULmax and wSULpeak) as well as TBR parameters predicted three-year survival, and some of the TBR parameters predicted which patients survived after five years. Considerably more metabolic parameters extracted from the three- and six-month follow-up scans predicted three-year and five-year survival, the researchers stated.
In addition, on the three- and six-month follow-up PET/CT scans, a higher total number of lesions detected in general and a higher number in the abdomen and pelvis predicted worse three-year and five-year survival rates, the researchers found.
"All of the most predictive parameters for [overall survival] were derived from the 3-month follow-up study," the authors noted.
The main limitations of the study include its retrospective nature and the relatively small sample size, the authors stated. They also noted that this study was not designed to evaluate the extent of patient response to specific immunotherapy treatments.
Ultimately, the study shows that F-18 FDG-PET/CT imaging metrics can provide promising prognostic information for making clinical decisions and may serve as new tools for better stratification and selection of patients with advanced melanoma, the authors suggested.
"Future prospective studies are required on a larger cohort of patients to confirm our findings," the group concluded.