PET imaging can show whether patients with melanoma or non-small cell lung cancer (NSCLC) will respond to immunotherapy treatment, according to a study published November 1 in Annals of Oncology.
Dutch researchers performed PET imaging with an immunotherapy drug combined with zirconium-89 (Zr-89) (so-called "immuno-PET") after they received treatment. They found the radiotracer revealed all tumor sites in patients, as well as whether their tumors were responding to the treatment.
"Zr-89 pembrolizumab uptake in tumor lesions correlates not only with response to therapy, but also with [progression-free survival] and [overall survival]," wrote first author Dr. Iris Kok of University of Groningen in the Netherlands.
The finding marks a major advance in evaluating immunotherapy, as so far, there are no effective noninvasive methods for determining how well these expensive treatments work, or if they are effective at all in some cancer patients, according to the authors.
Immune checkpoint inhibitors such as pembrolizumab (brand name Keytruda) are monoclonal antibodies that block signals from a protein called programmed cell death protein-1 (PD-1). PD-1 is found on the surface of cancer cells and emits signals that keep T cells from attacking cancer. When PD-1 is blocked, however, the body's natural immune system receives a boost to attack the cancer.
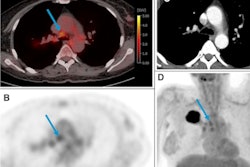
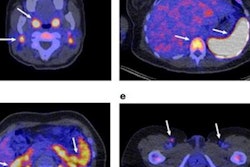
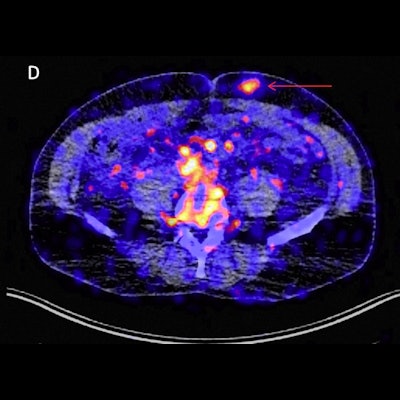
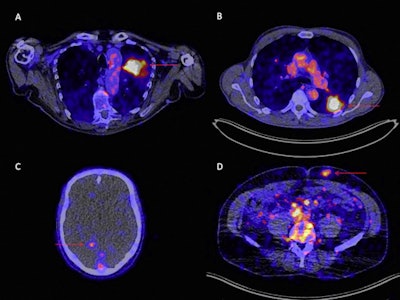 Zr-89 pembrolizumab-PET/CT image examples at day 7 of tumor lesions in various patients. Red arrows indicate tumor lesions. Images A and B are scaled 0-8 SUV, images C and D are scaled 0-5 SUV. A) Tumor lesion left upper lobe, NSCLC. B) Pulmonary metastasis, melanoma. C) Brain metastasis, melanoma (SUVmax 4.20). D) Abdominal wall metastasis, melanoma (SUVmax 5.02).
Zr-89 pembrolizumab-PET/CT image examples at day 7 of tumor lesions in various patients. Red arrows indicate tumor lesions. Images A and B are scaled 0-8 SUV, images C and D are scaled 0-5 SUV. A) Tumor lesion left upper lobe, NSCLC. B) Pulmonary metastasis, melanoma. C) Brain metastasis, melanoma (SUVmax 4.20). D) Abdominal wall metastasis, melanoma (SUVmax 5.02).In previous studies, the German group showed that the uptake of Zr-89 pembrolizumab-PET revealed tumors in mice. In this study, they sought to assess how effective it is in patients with melanoma and NSCLC.
The researchers recruited 18 patients with advanced or metastatic melanoma or NSCLC from two centers in the Netherlands. Patients first received Zr-89 pembrolizumab infusions and then up to three PET scans on days 2, 4, and 7. Tumor uptake of Zr-89 pembrolizumab was calculated as maximum standardized uptake value (SUVmax). Normal organ uptake was calculated as SUVmean and tumor response was assessed according to RECIST v1.1.
The study authors found the optimal dose of pembrolizumab was 5 mg, and the optimal time point for PET scanning was on day 7. The tumor SUVmax did not differ between melanoma and NSCLC (4.9 and 6.5, P = 0.49). In addition, tumor uptake of Zr-89 pembrolizumab correlated with tumor response (Ptrend = 0.014) and progression-free (p = 0.0025) and overall survival (p = 0.026), the researchers wrote.
"Zr-89 pembrolizumab-PET imaging is a safe and non-invasive imaging modality for whole-body visualization of PD-1 and pembrolizumab biodistribution," the group stated.
The authors noted they were unable to study the relationship between uptake and patient outcome in melanoma and NSCLC patients based on primary tumor subtypes due to a limited sample size. Hence, a future study might also include immune checkpoint inhibitor tumor uptake as a key treatment response factor, they wrote.
"These findings require validation in larger studies to prove their impact on patient selection for PD-1 blockade," the researchers concluded.