Radiopharmaceutical developer Navidea Biopharmaceuticals said that interim data from its phase IIB study for technetium-99m tilmanocept (TCT) in patients with rheumatoid arthritis (RA) shows that the radiopharmaceutical provides quantitative imaging in healthy controls as well as in patients with active RA.
In this third arm of its clinical study, a pilot of the upcoming phase III trial, Navidea assessed the ability of TCT to provide an early indicator of treatment efficacy in RA patients. Specifically, the third arm evaluated the magnitude of TCT signal change localized to RA-involved joints in patients before and after treatment with an antitumor necrosis factor (TNF) alpha treatment. Researchers sought to determine whether the change in localization, if any, could serve as an early, quantifiable predictor of treatment efficacy.
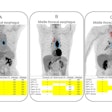
A total of 15 patients with active moderate-to-severe RA were included in the phase IIB analysis, each of whom were set to begin a new or first-time treatment regimen with an anti-TNF alpha therapy. Whole-body and hand/wrist planar gamma camera images were obtained at baseline prior to new treatment, then again at five weeks after therapy initiation, and again at 12 weeks on eight of the 15 patients.
The remaining seven participants received baseline and five-week scans only at the time of analysis. A panel of established clinical assessments was performed at each time point as well. Navidea said that the results showed the following:
- TCT imaging from baseline to week five predicted clinical outcome at 12 weeks in seven out of eight participants. The outlier had undergone a change in treatment regime while enrolled in the trial that may have impacted the trajectory of the clinical response.
- Combined data from all 15 subjects in the third arm suggest a range of more than one order of magnitude (> 10-fold) for calculated global TCT uptake values in joints with RA-involved inflammation.
- In the study participants with 12-week follow up data available, global TCT signals declined by an average of 58% from baseline to week five in those who responded significantly to anti-TNF alpha treatment by week 12. In patients who did not have a significant clinical response to anti-TNF alpha treatment by week 12, TCT signals increased by an average of 79% from baseline to five weeks. The preliminary results indicate marked changes in TCT global uptake values by week five are in agreement with clinical efficacy evaluations made at week 12 of treatment, Navidea said.
- The range of global TCT signal readout combined with the low variability of imaging signal quantification established in the first and second arms corroborate the idea that clinically meaningful changes in signal localization can be detected.
All told, the data supports Navidea's hypotheses that TCT imaging can provide quantifiable imaging assessment of RA-involved joints, according to the vendor. Doing so allows for early prediction of clinical response as well as longitudinal monitoring of clinical status, the firm said.
The results will spur Navidea to speak with the U.S. Food and Drug Administration (FDA) about moving into the phase III trial later this year, the company added.