Researchers at the University of Pennsylvania have published the first clinical images acquired using the prototype PennPET Explorer, the second of two large axial field-of-view (FOV) whole-body PET imagers developed by the U.S.-based Explorer Consortium.
The proof-of-concept studies showed that the PennPET Explorer can produce higher quality images in shorter times and offer far greater versatility than state-of-the art commercial PET scanners (Journal of Nuclear Medicine, September 27, 2019).
 Members of the University of Pennsylvania team of scientists and engineers who developed and evaluated the PennPET Explorer. All images courtesy of Joel Karp.
Members of the University of Pennsylvania team of scientists and engineers who developed and evaluated the PennPET Explorer. All images courtesy of Joel Karp.The PennPET Explorer is based on a digital silicon photomultiplier developed by Philips. The prototype configuration has three rings -- each consisting of 18 detector modules -- and an axial FOV of 64 cm. The scanner has a spatial resolution of 4.0 mm and time-of-flight resolution of 250 picoseconds -- leading to state-of-the-art imaging performance.
The Perelman School of Medicine researchers are continuing to develop the scanner's design, increasing the number of rings from three to six, thereby expanding the axial FOV to 140 cm, as well as integrating an in-line CT scanner. They are basing their designs on established technologies and manufacturing procedures to ensure that the PennPET Explorer can be developed into a commercial modality in the future.
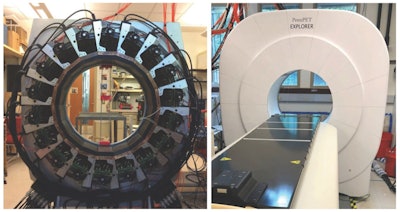 The PennPET Explorer scanner under development (left) and in prototype form with three rings (right), as used for initial human imaging evaluation.
The PennPET Explorer scanner under development (left) and in prototype form with three rings (right), as used for initial human imaging evaluation.Extended axial FOV PET scanners offer many advantages over existing PET systems. The PennPET Explorer, with its high sensitivity of 55 kcps/MBq, enables dynamic whole-body imaging with high temporal resolution, as well as imaging with decreased radiotracer dose or shorter scan times, without compromising image quality.
Whole-body coverage also permits kinetic analysis of lesions beyond a standard axial FOV, ensuring the inclusion of large vascular structures for input functions. Finally, the scanner can image a wider range of isotopes, including longer-lived radiotracers that allow study of slower biological processes and applications such as cell tracking. These capabilities could expand PET utilization to study diseases and medical conditions not currently interrogated with PET.
Initial clinical tests
Senior author Joel Karp and colleagues conducted their first human studies with three groups of individuals to test various capabilities of the PennPET Explorer. Participants included five healthy volunteers, three clinical patients, and two research subjects participating in PET studies using investigational radiotracers.
Healthy volunteers underwent F-18 FDG scans with a commercial PET/CT scanner followed by PennPET scans from head to abdomen. To simulate shorter scans, the researchers subsampled scan data. Comparison of 16-minute scans showed superior image quality in the PennPET scan. A subsampled 2-minute PennPET image showed comparable, if not better, quality to the 16-minute clinical scan.
To demonstrate the potential for dynamic whole-body imaging, two healthy subjects received bolus injections of F-18 FDG during an hour of dynamic imaging on the PennPET Explorer. The researchers also performed delayed scans, up to 10 half-lives after F-FDG injection, to study late kinetics and the ability to ability to image at low activity.
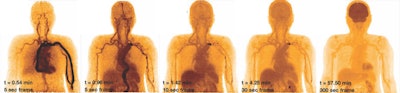 Dynamic study of a subject with bolus injection of F-18 FDG. Images at different time points over one hour illustrate the temporal uptake of FDG, moving from the circulatory system to the organ systems. Such a dynamic study is only possible using a scanner with a long axial FOV.
Dynamic study of a subject with bolus injection of F-18 FDG. Images at different time points over one hour illustrate the temporal uptake of FDG, moving from the circulatory system to the organ systems. Such a dynamic study is only possible using a scanner with a long axial FOV.In a patient with metastatic colon cancer, F-18 FDG PET images acquired on the PennPET Explorer showed more accurate delineation of disease than those taken using a standard PET scanner. This included more conspicuous perihepatic disease and an FDG-avid lymph node only visible on the PennPET image.
The team also examined a patient with metastatic neuroendocrine cancer involved in a gallium-68 DOTATATE PET study. A PennPET image using one-fifth the activity of the clinical PET scan showed comparable diagnostic quality to the clinical image, suggesting that the PennPET Explorer could offer a more practical option for this high-cost radiotracer.
In the two participants enrolled in separate research studies, one subject was imaged on the PennPET Explorer, from the vertex to the lower abdomen, two hours after injection of F-18 NOS, an imaging agent that targets inflammation. PennPET-acquired images revealed unexpected ocular uptake, which was excluded from the FOV of the standard research scan.
Another study imaged F-18 fluorotriopride, an agent for the dopamine D3 receptor, in the upper abdomen after the patient ate a fatty meal to stimulate gallbladder emptying. Images showed mild gallbladder emptying over time, highlighting potential uses for the PennPET Explorer in dosimetry studies.
"Our clinical studies with the prototype PennPET Explorer demonstrated excellent image quality and potential for imaging with lower activity and shorter scan duration, as well as demonstrating the potential for very delayed imaging and the measurement of multiorgan kinetics," wrote the authors.
Karp told Physics World that the completed scanner is being moved to another imaging facility in the school of medicine and will be operational in early 2020.
"Our focus will be on expanding the research PET program here by leveraging its unique capabilities. We are planning to undertake many possible studies that can take advantage of the larger axial FOV," he explained. "We will also be performing the performance and compliance tests to enable us to submit a 510(k) application to the [U.S. Food and Drug Administration (FDA)]. FDA compliance will enable us to eventually perform a wider variety of clinical studies, including pediatric studies with our colleagues at the Children's Hospital of Philadelphia."
Cynthia E. Keen is a freelance journalist specializing in medicine and healthcare-related innovations.
© IOP Publishing Limited. Republished with permission from Physics World, a website that helps scientists working in academic and industrial research stay up to date with the latest breakthroughs in physics and interdisciplinary science.