When scanning patients with suspected heart disease, the last thing you want is a bunch of incidental findings like lung nodules that are almost certainly not cancer. No really. Because finding them generally does more harm than good, according to a cardiac imaging expert who spoke last week at the British Institute of Radiology President's Conference in London.
There's an easy way to find fewer incidentals without avoiding a proper search for them -- all while improving image quality and dropping the dose, according to Dr. Matthew Budoff.
For the vast majority of patients getting a coronary CT angiography (CTA) scan, following up incidental findings leads not to a lifesaving lung cancer diagnosis but rather to more scans, higher costs, and increased morbidity and mortality.
"Screening just because you can, or just because the patient is already on the table, or just because we can expand the field-of-view doesn't mean that we're doing much good," said Budoff, who is a professor of medicine at the University of California School of Medicine, Los Angeles. "You're scanning thousands of patients to find one isolated person [with cancer]. And along the way there are costs."
Budoff's views, while well supported by examples in the literature he cites, are bound to raise eyebrows among radiologists who consider incidental findings an unavoidable but entirely managable part of CT imaging. Moreover, a number of studies focusing on other anatomic regions have found that few incidental findings are actually followed up, and that findings referred for additional scrutiny can be managed at a reasonable cost.
Fleischner Society scanfest
The 2005 Fleischner Society guidelines were the first to offer a clear blueprint for the follow-up of incidentally detected noncalcified lung nodules 4 to 6 mm or larger (Radiology, November 2005, Vol. 237:2, pp. 395-400). In the case of incidentally detected lung nodules 8 mm or larger, for example, the guidelines recommend repeat CT scans at three, nine, and 24 months.
"They're going to get five follow-up scans for a nodule that was seen incidentally and most likely is not cancer -- statistically 99.99% of lung nodules are not cancer," Budoff said. "And there's a 20% chance they're going to get another five CT scans as the result of a subsequent follow-up. If another nodule develops, they might get 10 follow-up CT scans if we follow these criteria -- all for seeing a small incidental speck in the lung fields."
Minimize field-of-view
Most CT scanner manufacturers now offer bowtie filters, which reduce the maximum field-of-view (FOV) allowable while cutting the radiation dose, Budoff said.
For example, using protocol parameters of 600 mA, 120 kV, and a heart rate of 60 bpm, a small cardiac bowtie filter (standard 25-cm FOV) permits cardiac images to be acquired at dose-length product (DLP) of 474 (8.0 mSv), Budoff said. A medium bowtie filter (allowing reconstruction to 36-cm FOV) affords a dose of 791.4 DLP (13.4 mSv).
From there, expanding the FOV to the entire thorax for screening of other important findings (lung, breast, axilla, mediastinum, and spine) would actually boost the radiation dose by 67.5%, he said.
Since CT is not an optimal way to evaluate breast tissue, the radiologist shares responsibility for reporting those incidental findings, he said, adding that misreading breast tissue or lung tissue generates more liability than if the radiologist had never evaluated it.
The heart fits in a 25-cm field-of-view, permitting the use of a small bowtie filter in medium-sized and sometimes even larger patients, Budoff said. "We're only worried about the heart," he said.
Of course, more anatomic coverage is often needed, Budoff said. Sometimes the radiologist needs to look for aortic dissection or pulmonary embolism, but at that point it's not a heart study.
"What I call a cardiac CT always includes the great vessels, the myocardium, the left and right atrium, and pericardium," Budoff said. "I'm never only interested in the coronary anatomy. But in cardiac CT, I'm never interested in the lung. If I'm interested in the lungs, I can do a dedicated lung scan for an additional 0.7 mSv of radiation and have it interpreted separately and done properly."
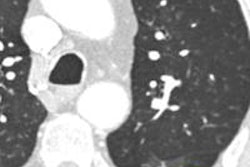
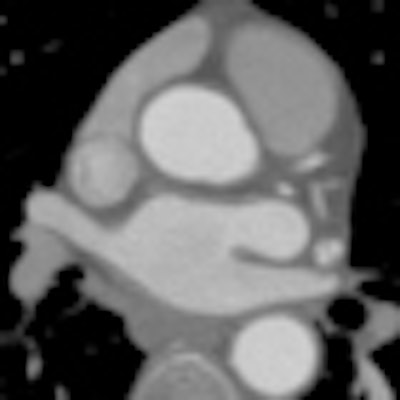
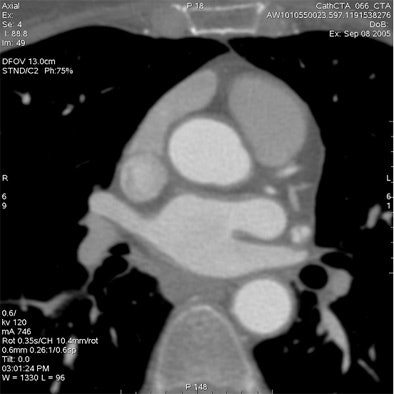 Which image would you rather be responsible for? Small 13-cm FOV (above) provides the best cardiac image quality at the lowest radiation dose compared with medium 25-cm FOV coronary CTA image (below). Thoracic image (bottom) maximizes anatomic coverage but at the cost of additional radiation and potentially more incidental findings. All images courtesy of Dr. Matthew Budoff.
Which image would you rather be responsible for? Small 13-cm FOV (above) provides the best cardiac image quality at the lowest radiation dose compared with medium 25-cm FOV coronary CTA image (below). Thoracic image (bottom) maximizes anatomic coverage but at the cost of additional radiation and potentially more incidental findings. All images courtesy of Dr. Matthew Budoff.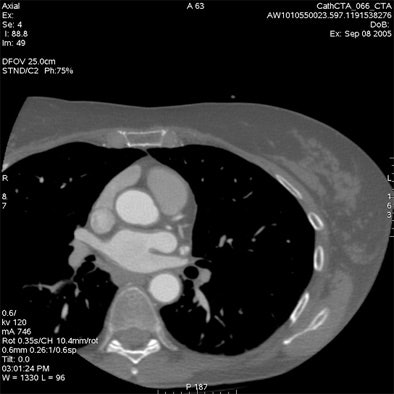
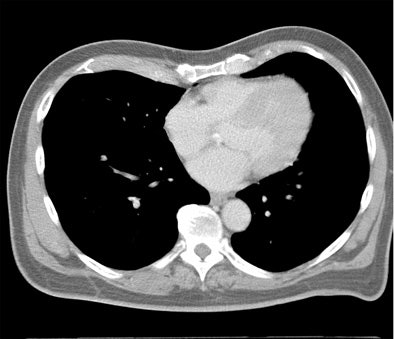
For most patients, following the principles of ALARA (dose as low as reasonably achievable), confining the field-of-view, and using a small bowtie filter for all patients undergoing cardiac scanning has the best resolution and evidence of efficacy, Budoff said.
"So I would suggest that sometimes less is more," he said. "Sometimes we want to do a cardiac CT and just do a cardiac CT."
That doesn't mean ignoring what you do see, however. Whenever extracardiac pathology is visible, a radiologist should evaluate it, Budoff said.
High-risk patients
In high-risk patients, such as older long-term smokers, the lungs often harbor important findings, Budoff said. The recent National Lung Cancer Screening Trial (NLST), halted on November 4, 2010, owing to its excellent results and presented at the 2010 RSNA meeting in Chicago, showed the benefits of low-dose screening CT in a high-risk population.
"NLST announced this was a very positive study that was stopped early because of the benefit of CT imaging, which was tremendous," Budoff said. "It's one of the only things we have that shows if you have some type of imaging study, patients actually live longer -- maybe [only] mammography and ultrasound screening for abdominal aortic aneurysm can join this very small club of ways to reduce mortality by scanning."
NLST examined 53,500 older men and women with a cigarette smoking history of at least 30 pack years. In all, 354 participants died in the CT arm of the study compared with 442 in the chest x-ray group, meaning 88 lives were saved per 53,500 participants. Thus, the number of patients needed to scan to save a single life was 608.
However, most coronary CTA patients aren't at such high risk for lung cancer, Budoff said. If the patients aren't older and they're not long-term smokers, for example, the number of patients that need to be scanned to save a single life is going to go way up.
"It might be one in 3,000, it might be one in 10,000 -- we don't know because we're never going to do that trial," he said.
Following lower-risk patients isn't cheap. Diagnostic costs include follow-up CT scans, consultations with a pulmonologist, biopsy, PET scans, bronchoscopy, and even surgery.
In 2003, Mahadevia and colleagues showed that costs might be as high as $2.3 million for each quality-adjusted life year (QALY) for lung evaluations at CT (Journal of the American Medical Association, January 15, 2003, Vol. 289:3, pp. 313-322).
"If it's your life, it's probably worth it, but in general that's a pretty high societal cost to start screening for lung cancer," Budoff said.
Again in 2009, MacHaalany and colleagues examined 966 coronary CTA patients, 41.5% of whom had noncardiac findings, including 1.2% with significant incidental findings (Journal of the American College of Cardiology, October 13, 2009, Vol. 54:16, pp. 1533-1541).
After adjusting for age, incidental findings were not found to be an independent predictor of noncardiac death, Budoff said.
Still, the costs attending to them were substantial. The total direct cost associated with investigating incidental findings was $83,035. One patient was harmed as a result of follow-up, while no case had a documented benefit from an incidental finding, he said.
"So I would argue that we need to separate in our minds high-risk patients, smokers, former smokers, and older smokers from low-risk patients," Budoff said. I would suggest that the pretest probability for lung cancer is strongly tied to age. Keep your FOV small and make sure you read the study."
The results of Kim et al in Radiology (May 2010, Vol. 255:2, pp. 369-376) offer a striking picture of just how rare significant lung findings are, he said.
The study involved the acquisition of full thoracic images that were read twice: once in the full version and once in a limited field-of-view. Among 11,654 patients, the investigators found 36 cancers on full thoracic scans or about three cancers per 1,000 patients scanned. The mean diameter was 23 mm, and just 16 of 36 cancers were stage IA cancers, the most curable kind. As for the rest, half of the patients with advanced cancer died within 20 months, Budoff said.
- On cardiac large FOV = 19 cancers were visible (0.16%)
- On cardiac small FOV = 4 cancers were visible (0.03%)
Looking at the small field-of-view meant missing some cancers, Budoff said, but in light of the fact that only 16 cancers were resectable, and that many of the patients died despite having undergone a full apex-to-base thoracic CT scan, the small field-of-view didn't miss much, Budoff said.
"When you're going after a low-risk population, you're going to find a lot of cancers that, having found them, don't impact their outcomes," Budoff said.
Cardiac imaging isn't lung screening, he said. "At this point we're just scanning random patients and putting them through the scanner, and we're mostly going to find nodules with an absence of benefit."
Cardiac scans yield a low prevalence (< 1%) of significant disease, he said. Upward of 99.9% of nodules are not cancer, therefore false-positive.
Screening in low-risk populations leads to potential harm from radiation exposure, biopsies, and surgery, while markedly increasing costs -- to as much as $2.3 million per year of life saved.
"There's at least a theoretical harm from radiation," Budoff said. "There's measurable harm from biopsies and surgery. If we start doing PET/CT, it's going to raise the radiation dose and risk even more."
Another study showed that the discovery of a lung nodule increased anxiety to physicians and patients, worsening the quality of life, he said, and the Fleischner Society guidelines themselves state that screening leads to perpetual testing in many patients.
"Less is more," Budoff said. "We keep the FOV small to reduce radiation and to reduce incidental nodules in a low-risk population where we know the yield is going to be zero or close to zero. There's less follow-up of studies and better image quality of the heart. There's less patient and physician anxiety, and it will lead to lower radiation exposure in the long run for our patients."