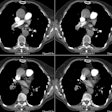
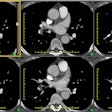
Aortic Coarctation:
View cases of aortic coarctation
1- Infantile: (Tubular Hypoplasia)
Infantile aortic coarctation is characterized by a long segment narrowing involving the proximal aortic arch at the origins of the great vessels. The proximal arch segment is defined as hypoplastic when its external diameter is less than 60% of that of the ascending aorta [9]. The corresponding limit for the distal arch is 50%, and the limit for the isthmus is 40% [9]. Patients present by 2 to 3 weeks of age with caudal cyanosis, and early demise (by about 3months). Tubular hypoplasia is the second most common cause of heart failure in newborns [7]. Associated congenital heart disease is common with a VSD found in 50% of cases. A patent ductus occurs in one-third of patients, but is required to supply blood to the descending aorta beyond the area of narrowing. Prostaglandin E is administered in order to keep the ductus patent until surgical repair can be performed [6].
On CXR there is cardiomegally with right atrial and right ventricular enlargement, coupled with pulmonary venous congestion.
2- Adult (Focal) aortic coarctation:
Clinical:
Adult coarctation is more common than the infantile form and is
characterized by a focal narrowing of the aorta just distal or
proximal to the ligamentum (ductus) arteriosus. It is the most
common congenital anomaly of the aorta- occurring in 0.4% of live
births and in 7% of patients with congenital heart disease [10].
Coarctation is only rarely associated with a right aortic arch,
with a reported prevalence of only 0.1% [11]. Although referred to
as an adult lesion, many patients are detected in late childhood
or adolescence [5]. Patients present with a murmur (young
patients), headache (due to hypertension which is
characteristically systolic and involves the upper extremities),
diminished lower extremity pulses, a radial artery to femoral
artery pulse delay, and claudication; although in many cases the
condition is well tolerated and the patient is asymptomatic. The
development of collateral circulation may also result in
relatively good peripheral pulses. Males are affected 1.5 to 4
times more commonly than females [1,6,9].
The condition is associated with a bicuspid aortic valve (22% to
50% of patients), Turner's syndrome (2% of cases, but
approximately 15-36% of patients with Turner's syndrome are
affected [7,10]), and cerebral berry aneurysms (up to 10% of cases
[9]). An association with Shone complex (LV outflow tract
obstruction and parachute mitral valve) has also been reported
[9]. Other associations include PHACES syndrome (posterior fossa
malformations, hemangiomas, arterial anomalies, cardiac defects,
eye abnormalities, sternal cleft, and supraumbilical raphe),
Williams-Beuren syndrome, and Alagille syndrome [10].
Non-treated aortic coarctation has a poor prognosis with a
reported mortality of 75% by 46 years of age [9]. CHF is the most
common cause of death, followed by aortic rupture, endocarditis,
and intracranial hemorrhage [9]. Studies have shown that the age
at coarct repair is a significant predictor of morbidity, with
worse outcomes in patients who were older at the time of repair
[10]. The indications for surgical repair are based upon the
demonstration of a significant gradient across the stenosis
(greater than or equal to 20 mmHg, a severe narrowing (greater
than 50%), or a gradient of less than 20 mmHg, but the
demonstration of significant collateral circulation [4,10].
Although measuring the pressure gradient across the narrowed
segment has been used routinely to determine the hemodynamic
significance of a coarctation, the presence of collateral vascular
channels can reduce the measured pressure gradient and introduce
errors in estimating the severity of the obstruction [5].
Data suggest that when possible, resection with end-to-end
anastomosis is the preferred treatment technique in neonates and
infants with improved late outcomes and a decreased risk for
reintervention compared to alternative techniques [10]. Surgical
complications include recurrent laryngeal nerve paralysis, phrenic
nerve injury, chylothorax, and paraplegia [10].
Balloon angioplasty has fallen out of favor for repair outside of
infancy [10]. The rate of restenosis is greater with balloon
angioplasty, as is the incidence of aneurysm or pseudoaneurysm,
but rupture of the aorta from the procedure is rare [2].
Stent placement is being increasingly utilized [10], but is
primarily reserved for patients who have re-coarctation from
following a prior repair [8] and adults [10]. Other indications
for stenting include unfavorable anatomy for balloon angioplasty
(long segment narrowing) and those patients that are poor surgical
candidates [8]. The use of stents is generally avoided in children
due to the large size of the delivery system and the need for
repeat dilatation as the child grows [8].
A differential consideration includes pseudocoartation which is a rare anomaly that represents a "kinking" or "buckling" of the aorta at the ductus/ligamentum arteriosum which is seen in older patients, but produces no pressure gradient (< 25mm Hg) and no collateral vessel formation [11].
X-ray:
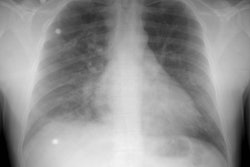
CXR: CXR findings include dilatation of the ascending aorta, left ventricular enlargement, and a "figure 3" sign which represents an indentation on the lateral wall of the descending aorta with post stenotic dilatation. The "figure 3" sign is noted in 50 to 66% of cases in adult's [7]. The "reverse figure of three" sign is seen on the LAO projection during barium swallow [7]. Soft tissue densities can be identified behind the sternum and represent dilated internal mammary arteries. There can be loss of the normal appearing aortic knob due to loss of the superior margin of the vessel secondary to dilatation/ enlargement of the left subclavian artery. Inferior rib notching is rarely seen before age 5 years, but is seen in most adults over the age of 20. Notching involves the posterior 3rd through 9th ribs, but it is usually most apparent in the upper ribs. It occurs due to pressure erosion by dilated intercostal arteries which serve as collaterals between the internal mammary arteries and the descending aorta. The 1st and 2nd ribs are not involved because the first two intercostal arteries arise from the thyro/costocervical trunk which is located proximal to coarctation (hence these vessels do not serve as collaterals). Differential considerations for inferior rib notching include: Coarctation, large vessel vasculitis, and Neurofibromatosis. Unilateral rib notching suggests an arch anomaly such as an anomalous subclavian artery originating below the level of the coarctation. In this situation, the ribs on the affected side will not demonstrate notching- for instance, in a patient with a left arch, but an aberrant right subclavian artery arising distal to the coarctation, only unilateral left sided notching will be identified.
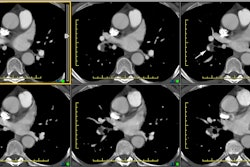
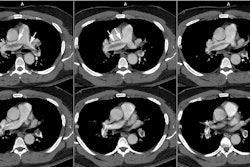
Computed tomography: CT axial imaging is limited in its ability to depict the anatomy of the coarctation, although with helical scanning and reconstruction images, this may not longer apply.
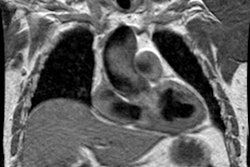
Magnetic resonance imaging: MR imaging in the sagittal or sagittal oblique plane will usually demonstrate the lesion very accurately. Collateral vessels can be identified and phase-contrast images can also be used to detect collateral flow. Cine GRE images will demonstrate the jet of turbulent flow through the area of narrowing as an area of signal void and the length of the signal void has been correlated with the severity of the stenosis. Velocity-encoded cine MR can be used to measure blood flow in the descending aorta just distal to the coarctation and at the level of the diaphragm- the difference in flow will represent the contribution of collateral vessels. Normals have an average decrease in blood flow between these two points of 8%. In patients with aortic coarctation, there is an average increase in flow of 80%. [3]
REFERENCES:
(1) AJR 1997; Raymond GS, et al. Congenital thoracic lesions that mimic neoplastic disease on chest radiographs of adults. 168: 763-769 (No abstract available)
(2) J Thorac Imaging 1995; 10(1): 36-42
(3) Magn Reson Imaging Clin N Am 1996; 4(2): 217-235
(4) Cardiology Clinics 1988; Fixler DE. Coarctation of the aorta. 6(4): 561-571 (Review- no abstract available)
(5) AJR 1997; Julsrud PR, et al. Coarctation of the aorta: Collateral flow assessment with phase-contrast MR angiography. 169: 1735-42
(6) Pediatric Clinics of North America 1999; Fedderly RT. Left ventricular outflow obstruction. 46 (2): 369-384
(7) Radiographics 2007; Ferguson EC, et al. Classic imaging signs of congenital cardiovascular abnormalities. 27: 1323-1334
(8) Radiology 2008; Gaca AM, et al. Repair of congenital heart disease: a primer- part 2. 248: 44-60
(9) Radiographics 2010; Kimura-Hayama ET, et al. Uncommon
congenital and acquired aortic diseases: role of multidetector CT
angiography. 30: 79-98
(10) J Cardiovasc Comput Tomogr 2016; Nance JW, et al.
Coarctation of the aorta in adolescents and adults: a review of
clinical featuers and CT imaging. 10: 1-12
(11) Radiographics 2017; Hanneman K, et al. Congenital variants and anomalies of the aortic arch. 37: 32-51